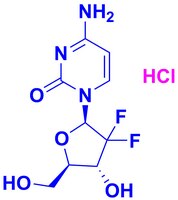
504594 Sigma-AldrichGemcitabine, HCl - CAS 122111-03-9 - Calbiochem
Synonyms: Ribonucleotide Reductase Inhibitor II, RNR Inhibitor II, 2ʹ-Deoxy-2ʹ,2ʹ-difluorocytidine, HCl, 2ʹ,2ʹ-Difluoro-2ʹ-deoxycytidine, HCl, 4-Amino-1-((2R,5S)-3,3-difluoro-5-(hydroxymethyl)tetrahydrofuran-2-yl)pyrimidin-2(1H)-one, HCl, LY188011, dFdC, dFdCyd
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| CAS # | Empirical Formula |
|---|---|
| 122111-03-9 | C₉H₁₁F₂N₃O₄ • HCl |
Pricing & Availability
| Catalogue Number | Availability | Packaging | Qty/Pack | Price | Quantity | |
|---|---|---|---|---|---|---|
| 5.04594.0001 |
|
Glass bottle | 50 mg |
|
— |
| Product Information | |
|---|---|
| CAS number | 122111-03-9 |
| Form | White powder |
| Hill Formula | C₉H₁₁F₂N₃O₄ • HCl |
| Chemical formula | C₉H₁₁F₂N₃O₄ • HCl |
| Hygroscopic | Hygroscopic |
| Reversible | N |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | DNA |
| Secondary target | DNA polymerase |
| Purity | ≥99% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 5.04594.0001 | 04055977243895 |
Documentation
Gemcitabine, HCl - CAS 122111-03-9 - Calbiochem SDS
| Title |
|---|
Gemcitabine, HCl - CAS 122111-03-9 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 504594 |
References
| Reference overview |
|---|
| Hung, S.W., et al. 2012. Cancer Lett. 320, 138. Fowler, J.D., et al. 2008. J. Biol. Chem. 283, 15339. Mackey, J.R., et al. 1998. Cancer Res. 58, 4349. Eda, H., et al. 1998. Cancer Res. 58, 1165. Burris III, H.A., et al. 1997. J. Clin. Oncol. 15, 2403. Heinemann, V., et al. 1992. Cancer Res. 52, 533. Hertel, L.W., et al. 1990. Cancer Res. 50, 4417. |