miR-142-5p and miR-130a-3p are regulated by IL-4 and IL-13 and control profibrogenic macrophage program.
Su, S; Zhao, Q; He, C; Huang, D; Liu, J; Chen, F; Chen, J; Liao, JY; Cui, X; Zeng, Y; Yao, H; Su, F; Liu, Q; Jiang, S; Song, E
Nature communications
6
8523
2015
Afficher le résumé
Macrophages play a pivotal role in tissue fibrogenesis, which underlies the pathogenesis of many end-stage chronic inflammatory diseases. MicroRNAs are key regulators of immune cell functions, but their roles in macrophage's fibrogenesis have not been characterized. Here we show that IL-4 and IL-13 induce miR-142-5p and downregulate miR-130a-3p in macrophages; these changes sustain the profibrogenic effect of macrophages. In vitro, miR-142-5p mimic prolongs STAT6 phosphorylation by targeting its negative regulator, SOCS1. Blocking miR-130a relieves its inhibition of PPARγ, which coordinates STAT6 signalling. In vivo, inhibiting miR-142-5p and increasing miR-130a-3p expression with locked nucleic acid-modified oligonucleotides inhibits CCL4-induced liver fibrosis and bleomycin-induced lung fibrosis in mice. Furthermore, macrophages from the tissue samples of patients with liver cirrhosis and idiopathic pulmonary fibrosis display increased miR-142-5p and decreased miR-130a-3p expression. Therefore, miR-142-5p and miR-130a-3p regulate macrophage profibrogenic gene expression in chronic inflammation. | | 26436920
 |
Histone modifications are responsible for decreased Fas expression and apoptosis resistance in fibrotic lung fibroblasts.
Huang, SK; Scruggs, AM; Donaghy, J; Horowitz, JC; Zaslona, Z; Przybranowski, S; White, ES; Peters-Golden, M
Cell death & disease
4
e621
2013
Afficher le résumé
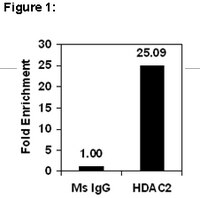
Although the recruitment of fibroblasts to areas of injury is critical for wound healing, their subsequent apoptosis is necessary in order to prevent excessive scarring. Fibroproliferative diseases, such as pulmonary fibrosis, are often characterized by fibroblast resistance to apoptosis, but the mechanism(s) for this resistance remains elusive. Here, we employed a murine model of pulmonary fibrosis and cells from patients with idiopathic pulmonary fibrosis (IPF) to explore epigenetic mechanisms that may be responsible for the decreased expression of Fas, a cell surface death receptor whose expression has been observed to be decreased in pulmonary fibrosis. Murine pulmonary fibrosis was elicited by intratracheal injection of bleomycin. Fibroblasts cultured from bleomycin-treated mice exhibited decreased Fas expression and resistance to Fas-mediated apoptosis compared with cells from saline-treated control mice. Although there were no differences in DNA methylation, the Fas promoter in fibroblasts from bleomycin-treated mice exhibited decreased histone acetylation and increased histone 3 lysine 9 trimethylation (H3K9Me3). This was associated with increased histone deacetylase (HDAC)-2 and HDAC4 expression. Treatment with HDAC inhibitors increased Fas expression and restored susceptibility to Fas-mediated apoptosis. Fibroblasts from patients with IPF likewise exhibited decreased histone acetylation and increased H3K9Me3 at the Fas promoter and increased their expression of Fas in the presence of an HDAC inhibitor. These findings demonstrate the critical role of histone modifications in the development of fibroblast resistance to apoptosis in both a murine model and in patients with pulmonary fibrosis and suggest novel approaches to therapy for progressive fibroproliferative disorders. | Western Blotting | 23640463
 |