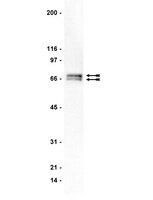
Nuclear translocation of cardiac G protein-Coupled Receptor kinase 5 downstream of select Gq-activating hypertrophic ligands is a calmodulin-dependent process.
Gold, JI; Martini, JS; Hullmann, J; Gao, E; Chuprun, JK; Lee, L; Tilley, DG; Rabinowitz, JE; Bossuyt, J; Bers, DM; Koch, WJ
PloS one
8
e57324
2013
Afficher le résumé
G protein-Coupled Receptors (GPCRs) kinases (GRKs) play a crucial role in regulating cardiac hypertrophy. Recent data from our lab has shown that, following ventricular pressure overload, GRK5, a primary cardiac GRK, facilitates maladaptive myocyte growth via novel nuclear localization. In the nucleus, GRK5's newly discovered kinase activity on histone deacetylase 5 induces hypertrophic gene transcription. The mechanisms governing the nuclear targeting of GRK5 are unknown. We report here that GRK5 nuclear accumulation is dependent on Ca(2+)/calmodulin (CaM) binding to a specific site within the amino terminus of GRK5 and this interaction occurs after selective activation of hypertrophic Gq-coupled receptors. Stimulation of myocytes with phenylephrine or angiotensinII causes GRK5 to leave the sarcolemmal membrane and accumulate in the nucleus, while the endothelin-1 does not cause nuclear GRK5 localization. A mutation within the amino-terminus of GRK5 negating CaM binding attenuates GRK5 movement from the sarcolemma to the nucleus and, importantly, overexpression of this mutant does not facilitate cardiac hypertrophy and related gene transcription in vitro and in vivo. Our data reveal that CaM binding to GRK5 is a physiologically relevant event that is absolutely required for nuclear GRK5 localization downstream of hypertrophic stimuli, thus facilitating GRK5-dependent regulation of maladaptive hypertrophy. | Western Blotting | | 23472081
 |
Toll-like receptors differentially regulate GPCR kinases and arrestins in primary macrophages.
Loniewski, Katie, et al.
Mol. Immunol., 45: 2312-22 (2008)
2008
Afficher le résumé
G-protein coupled receptor kinases (GRKs) and arrestins (ARRs) are ubiquitously distributed crucial signaling proteins that are critical in the regulation of responsiveness of G-protein coupled receptors (GPCRs). Toll-like receptors (TLRs) (class of pattern recognition receptors) play a vital role in macrophage biology and innate immunity. Because GPCR responsiveness is regulated in part by the expression levels of GRKs/ARRs, the focus of this work was to uncover potential cross-talk mechanisms between TLRs and GPCRs via regulation of GRK/ARR expression in primary mouse macrophages. We demonstrate here that activation of TLR2 and 4 (but not TLR3 and 7) significantly decrease ARR2 but not ARR3 protein levels in macrophages. Compared to this, activation of TLR2, 4, and 7 (but not TLR3) significantly decrease GRK5 and 6 protein levels. Surprisingly, GRK2 protein levels are markedly increased by TLR2, 3, 4 and 7. Mechanistically, expression of ARR2 and GRK5 are regulated at transcriptional as well as post-translational levels. Downregulation of GRK6 by LPS is regulated primarily at the post-translational level. TLR4-induced GRK2 level, however, is both transcriptionally and post-transcriptionally regulated. Our results demonstrate previously unknown crucial regulatory mechanisms that alter ARR/GRK expression levels in macrophages that might modify many, if not all, GPCR-mediated innate immune responses. | | Mouse | 18180038
 |
Phosphorylation and desensitization of human endothelin A and B receptors. Evidence for G protein-coupled receptor kinase specificity.
Freedman, N J, et al.
J. Biol. Chem., 272: 17734-43 (1997)
1997
Afficher le résumé
Although endothelin-1 can elicit prolonged physiologic responses, accumulating evidence suggests that rapid desensitization affects the primary G protein-coupled receptors mediating these responses, the endothelin A and B receptors (ETA-R and ETB-R). The mechanisms by which this desensitization proceeds remain obscure, however. Because some intracellular domain sequences of the ETA-R and ETB-R differ substantially, we tested the possibility that these receptor subtypes might be differentially regulated by G protein-coupled receptor kinases (GRKs). Homologous, or receptor-specific, desensitization occurred within 4 min both in the ETA-R-expressing A10 cells and in 293 cells transfected with either the human ETA-R or ETB-R. In 293 cells, this desensitization corresponded temporally with agonist-induced phosphorylation of each receptor, assessed by receptor immunoprecipitation from 32Pi-labeled cells. Agonist-induced receptor phosphorylation was not substantially affected by PKC inhibition but was reduced 40% (p < 0.03) by GRK inhibition, effected by a dominant negative GRK2 mutant. Inhibition of agonist-induced phosphorylation abrogated agonist-induced ETA-R desensitization. Overexpression of GRK2, -5, or -6 in 293 cells augmented agonist-induced ET-R phosphorylation approximately 2-fold (p < 0.02), but each kinase reduced receptor-promoted phosphoinositide hydrolysis differently. While GRK5 inhibited ET-R signaling by only approximately 25%, GRK2 inhibited ET-R signaling by 80% (p < 0.01). Congruent with its superior efficacy in suppressing ET-R signaling, GRK2, but not GRK5, co-immunoprecipitated with the ET-Rs in an agonist-dependent manner. We conclude that both the ETA-R and ETB-R can be regulated indistinguishably by GRK-initiated desensitization. We propose that because of its affinity for ET-Rs demonstrated by co-immunoprecipitation, GRK2 is the most likely of the GRKs to initiate ET-R desensitization. | | | 9211925
 |
Involvement of G protein-coupled receptor kinase 5 in homologous desensitization of the thyrotropin receptor.
Nagayama, Y, et al.
J. Biol. Chem., 271: 10143-8 (1996)
1996
Afficher le résumé
Homologous desensitization of G protein-coupled receptors involves agonist-dependent phosphorylation of receptors by G protein-coupled receptor kinases (GRKs). To identify GRK(s) that play a role in homologous desensitization of the thyrotropin (TSH) receptor, thyroid cDNA was amplified by polymerase chain reaction using degenerate oligonucleotide primers from highly conserved regions in GRK family. GRK5 is found in the predominant isoform expressed in the thyroid. Rat GRK5 cDNA was then isolated, which encodes a 590-amino acid protein with 95% homology to human and bovine homologs. Northern blot identified GRK5 mRNA of approximately 3, 8, and 10 kilobases with highest expression levels in lung > heart, kidney, colon > thyroid. In functional studies using a normal rat thyroid FRTL5 cells, overexpression of GRK5 by transfecting the plasmid capable of expressing the sense GRK5 RNA suppressed basal cAMP levels and augmented the extent of TSH receptor desensitization, whereas suppression of endogenous GRK5 expression by transfecting the antisense GRK5 construct increased basal cAMP levels and attenuated the extent of receptor desensitization. Although exogenously overexpressed GRK6 also enhanced TSH receptor desensitization, we conclude that GRK5, the predominant GRK isoform in the thyroid, appears to be mainly involved in homologous desensitization of the TSH receptor. | | | 8626574
 |
Monoclonal antibodies reveal receptor specificity among G-protein-coupled receptor kinases.
Oppermann, M, et al.
Proc. Natl. Acad. Sci. U.S.A., 93: 7649-54 (1996)
1996
Afficher le résumé
Guanine nucleotide-binding regulatory protein (G protein)-coupled receptor kinases (GRKs) constitute a family of serine/threonine kinases that play a major role in the agonist-induced phosphorylation and desensitization of G-protein-coupled receptors. Herein we describe the generation of monoclonal antibodies (mAbs) that specifically react with GRK2 and GRK3 or with GRK4, GRK5, and GRK6. They are used in several different receptor systems to identify the kinases that are responsible for receptor phosphorylation and desensitization. The ability of these reagents to inhibit GRK- mediated receptor phosphorylation is demonstrated in permeabilized 293 cells that overexpress individual GRKs and the type 1A angiotensin II receptor. We also use this approach to identify the endogenous GRKs that are responsible for the agonist-induced phosphorylation of epitope-tagged beta2- adrenergic receptors (beta2ARs) overexpressed in rabbit ventricular myocytes that are infected with a recombinant adenovirus. In these myocytes, anti-GRK2/3 mAbs inhibit isoproterenol-induced receptor phosphorylation by 77%, while GRK4-6-specific mAbs have no effect. Consistent with the operation of a betaAR kinase-mediated mechanism, GRK2 is identified by immunoblot analysis as well as in a functional assay as the predominant GRK expressed in these cells. Microinjection of GRK2/3-specific mAbs into chicken sensory neurons, which have been shown to express a GRK3-like protein, abolishes desensitization of the alpha2AR-mediated calcium current inhibition. The intracellular inhibition of endogenous GRKs by mAbs represents a novel approach to the study of receptor specificities among GRKs that should be widely applicable to many G-protein-coupled receptors. | | | 8755530
 |
Phosphatidylinositol 4,5-bisphosphate (PIP2)-enhanced G protein-coupled receptor kinase (GRK) activity. Location, structure, and regulation of the PIP2 binding site distinguishes the GRK subfamilies.
Pitcher, J A, et al.
J. Biol. Chem., 271: 24907-13 (1996)
1996
Afficher le résumé
The G protein-coupled receptor kinases (GRKs) phosphorylate agonist occupied G protein-coupled receptors and play an important role in mediating receptor desensitization. The localization of these enzymes to their membrane incorporated substrates is required for their efficient function and appears to be a highly regulated process. In this study we demonstrate that phosphatidylinositol 4, 5-bisphosphate (PIP2) enhances GRK5-mediated beta-adrenergic receptor (betaAR) phosphorylation by directly interacting with this enzyme and facilitating its membrane association. GRK5-mediated phosphorylation of a soluble peptide substrate is unaffected by PIP2, suggesting that the PIP2-enhanced receptor kinase activity arises as a consequence of this membrane localization. The lipid binding site of GRK5 exhibits a high degree of specificity and appears to reside in the amino terminus of this enzyme. Mutation of six basic residues at positions 22, 23, 24, 26, 28, and 29 of GRK5 ablates the ability of this kinase to bind PIP2. This region of the GRK5, which has a similar distribution of basic amino acids to the PIP2 binding site of gelsolin, is highly conserved between members of the GRK4 subfamily (GRK4, GRK5, and GRK6). Indeed, all the members of the GRK4 subfamily exhibit PIP2-dependent receptor kinase activity. We have shown previously that the membrane association of betaARK (beta-adrenergic receptor kinase) (GRK2) is mediated, in vitro, by the simultaneous binding of PIP2 and the betagamma subunits of heterotrimeric G proteins to the carboxyl-terminal pleckstrin homology domain of this enzyme (Pitcher, J. A., Touhara, K., Payne, E. S., and Lefkowitz, R. J. (1995) J. Biol. Chem. 270, 11707-11710). Thus, five members of the GRK family bind PIP2, betaARK (GRK2), betaARK2 (GRK3), GRK4, GRK5, and GRK6. However, the structure, location, and regulation of the PIP2 binding site distinguishes the betaARK (GRK2 and GRK3) and GRK4 (GRK4, GRK5, and GRK6) subfamilies. | | | 8798768
 |