Impact of a deletion of the full-length and short isoform of p75NTR on cholinergic innervation and the population of postmitotic doublecortin positive cells in the dentate gyrus.
Poser, R; Dokter, M; von Bohlen Und Halbach, V; Berger, SM; Busch, R; Baldus, M; Unsicker, K; von Bohlen Und Halbach, O
Frontiers in neuroanatomy
9
63
2015
Afficher le résumé
Analyses of mice carrying a deletion of the pan-neurotrophin receptor p75NTR have allowed identifying p75NTR as an important structural regulator of the hippocampus. Most of the previous analyses were done using p75NTR (ExIII) knockout mice which still express the short isoform of p75NTR. To scrutinize the role of p75NTR in the hippocampus, we analyzed adult and aged p75NTR (ExIV) knockout mice, in which both, the short and the full-length isoform are deleted. Deletion of these isoforms induced morphological alterations in the adult dentate gyrus (DG), leading to an increase in the thickness of the molecular and granular layer. Based on these observations, we next determined the morphological substrates that might contribute to this phenotype. The cholinergic innervation of the molecular and granular layer of the DG was found to be significantly increased in the knockout mice. Furthermore, adult neurogenesis in the DG was found to be significantly altered with increased numbers of doublecortin (DCX) positive cells and reduced numbers of apoptotic cells in p75NTR (ExIV) knockout mice. However, cell proliferation as measured by phosphohiston H3 (PH3) positive cell numbers was not affected. These morphological alterations (number of DCX-positive cells and increased cholinergic fiber densities) as well as reduced cell death in the DG are likely to contribute to the observed thickening of the granular layer in p75NTR (ExIV) knockout mice. In addition, Sholl-analysis of DCX-positive neurons revealed a higher dendritic complexity and could thus be a possible morphological correlate for the increased thickness of the molecular layer in p75NTR deficient animals. Our data clearly demonstrate that deletion of both, the short and the full-length isoform of p75NTR affects DG morphology, due to alterations of the cholinergic system and an imbalance between neurogenesis and programmed cell death within the subgranular zone. | | | 26074780
 |
Generation of a Retinoblastoma (Rb)1-inducible dominant-negative (DN) mouse model.
Tarang, S; Doi, SM; Gurumurthy, CB; Harms, D; Quadros, R; Rocha-Sanchez, SM
Frontiers in cellular neuroscience
9
52
2015
Afficher le résumé
Retinoblastoma 1 (Rb1) is an essential gene regulating cellular proliferation, differentiation, and homeostasis. To exert these functions, Rb1 is recruited and physically interacts with a growing variety of signaling pathways. While Rb1 does not appear to be ubiquitously expressed, its expression has been confirmed in a variety of hematopoietic and neuronal-derived cells, including the inner ear hair cells (HCs). Studies in transgenic mice demonstrate that complete germline or conditional Rb1 deletion leads to abnormal cell proliferation, followed by massive apoptosis; making it difficult to fully address Rb1's biochemical activities. To overcome these limitations, we developed a tetracycline-inducible TetO-CB-myc6-Rb1 (CBRb) mouse model to achieve transient and inducible dominant-negative (DN) inhibition of the endogenous RB1 protein. Our strategy involved fusing the Rb1 gene to the lysosomal protease pre-procathepsin B (CB), thus allowing for further routing of the DN-CBRb fusion protein and its interacting complexes for proteolytic degradation. Moreover, reversibility of the system is achieved upon suppression of doxycycline (Dox) administration. Preliminary characterization of DN-CBRb mice bred to a ubiquitous rtTA mouse line demonstrated a significant inhibition of the endogenous RB1 protein in the inner ear and in a number of other organs where RB1 is expressed. Examination of the postnatal (P) DN-CBRb mice inner ear at P10 and P28 showed the presence of supernumerary inner HCs (IHCs) in the lower turns of the cochleae, which corresponds to the described expression domain of the endogenous Rb1 gene. Selective and reversible suppression of gene expression is both an experimental tool for defining function and a potential means to medical therapy. Given the limitations associated with Rb1-null mice lethality, this model provides a valuable resource for understanding RB1 activity, relative contribution to HC regeneration and its potential therapeutic application. | | | 25755634
 |
Folate deficiency decreases apoptosis of endometrium decidual cells in pregnant mice via the mitochondrial pathway.
Liao, XG; Li, YL; Gao, RF; Geng, YQ; Chen, XM; Liu, XQ; Ding, YB; Mu, XY; Wang, YX; He, JL
Nutrients
7
1916-32
2015
Afficher le résumé
It is well known that maternal folate deficiency results in adverse pregnancy outcomes. In addition to aspects in embryonic development, maternal uterine receptivity and the decidualization of stromal cells is also very important for a successful pregnancy. In this study, we focused on endometrium decidualization and investigated whether apoptosis, which is essential for decidualization, was impaired. Flow cytometry and TUNEL detection revealed that apoptosis of mouse endometrium decidual cells was suppressed in the dietary folate-deficient group on Days 7 and 8 of pregnancy (Day 1 = vaginal plug) when decidua regression is initiated. The endometrium decidual tissue of the folate deficiency group expressed less Bax compared to the normal diet group while they had nearly equal expression of Bcl2 protein. Further examination revealed that the mitochondrial transmembrane potential (ΔΨm) decreased, and the fluorescence of diffuse cytoplasmic cytochrome c protein was detected using laser confocal microscopy in normal decidual cells. However, no corresponding changes were observed in the folate-deficient group. Western blotting analyses confirmed that more cytochrome c was released from mitochondria in normal decidual cells. Taken together, these results demonstrated that folate deficiency could inhibit apoptosis of decidual cells via the mitochondrial apoptosis pathway, thereby restraining decidualization of the endometrium and further impairing pregnancy. | | | 25781218
 |
Dysregulation of astrocyte extracellular signaling in Costello syndrome.
Krencik, R; Hokanson, KC; Narayan, AR; Dvornik, J; Rooney, GE; Rauen, KA; Weiss, LA; Rowitch, DH; Ullian, EM
Science translational medicine
7
286ra66
2015
Afficher le résumé
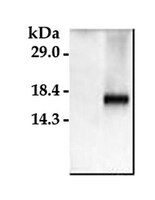
Astrocytes produce an assortment of signals that promote neuronal maturation according to a precise developmental timeline. Is this orchestrated timing and signaling altered in human neurodevelopmental disorders? To address this question, the astroglial lineage was investigated in two model systems of a developmental disorder with intellectual disability caused by mutant Harvey rat sarcoma viral oncogene homolog (HRAS) termed Costello syndrome: mutant HRAS human induced pluripotent stem cells (iPSCs) and transgenic mice. Human iPSCs derived from patients with Costello syndrome differentiated to astroglia more rapidly in vitro than those derived from wild-type cell lines with normal HRAS, exhibited hyperplasia, and also generated an abundance of extracellular matrix remodeling factors and proteoglycans. Acute treatment with a farnesyl transferase inhibitor and knockdown of the transcription factor SNAI2 reduced expression of several proteoglycans in Costello syndrome iPSC-derived astrocytes. Similarly, mice in which mutant HRAS was expressed selectively in astrocytes exhibited experience-independent increased accumulation of perineuronal net proteoglycans in cortex, as well as increased parvalbumin expression in interneurons, when compared to wild-type mice. Our data indicate that astrocytes expressing mutant HRAS dysregulate cortical maturation during development as shown by abnormal extracellular matrix remodeling and implicate excessive astrocyte-to-neuron signaling as a possible drug target for treating mental impairment and enhancing neuroplasticity. | Western Blotting | | 25947161
 |
Neuronal activity regulates remyelination via glutamate signalling to oligodendrocyte progenitors.
Gautier, HO; Evans, KA; Volbracht, K; James, R; Sitnikov, S; Lundgaard, I; James, F; Lao-Peregrin, C; Reynolds, R; Franklin, RJ; Káradóttir, RT
Nature communications
6
8518
2015
Afficher le résumé
Myelin regeneration can occur spontaneously in demyelinating diseases such as multiple sclerosis (MS). However, the underlying mechanisms and causes of its frequent failure remain incompletely understood. Here we show, using an in-vivo remyelination model, that demyelinated axons are electrically active and generate de novo synapses with recruited oligodendrocyte progenitor cells (OPCs), which, early after lesion induction, sense neuronal activity by expressing AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid)/kainate receptors. Blocking neuronal activity, axonal vesicular release or AMPA receptors in demyelinated lesions results in reduced remyelination. In the absence of neuronal activity there is a ∼6-fold increase in OPC number within the lesions and a reduced proportion of differentiated oligodendrocytes. These findings reveal that neuronal activity and release of glutamate instruct OPCs to differentiate into new myelinating oligodendrocytes that recover lost function. Co-localization of OPCs with the presynaptic protein VGluT2 in MS lesions implies that this mechanism may provide novel targets to therapeutically enhance remyelination. | | | 26439639
 |
Nuclear lamin stiffness is a barrier to 3D migration, but softness can limit survival.
Harada, T; Swift, J; Irianto, J; Shin, JW; Spinler, KR; Athirasala, A; Diegmiller, R; Dingal, PC; Ivanovska, IL; Discher, DE
The Journal of cell biology
204
669-82
2014
Afficher le résumé
Cell migration through solid tissue often involves large contortions of the nucleus, but biological significance is largely unclear. The nucleoskeletal protein lamin-A varies both within and between cell types and was shown here to contribute to cell sorting and survival in migration through constraining micropores. Lamin-A proved rate-limiting in 3D migration of diverse human cells that ranged from glioma and adenocarcinoma lines to primary mesenchymal stem cells (MSCs). Stoichiometry of A- to B-type lamins established an activation barrier, with high lamin-A:B producing extruded nuclear shapes after migration. Because the juxtaposed A and B polymer assemblies respectively conferred viscous and elastic stiffness to the nucleus, subpopulations with different A:B levels sorted in 3D migration. However, net migration was also biphasic in lamin-A, as wild-type lamin-A levels protected against stress-induced death, whereas deep knockdown caused broad defects in stress resistance. In vivo xenografts proved consistent with A:B-based cell sorting, and intermediate A:B-enhanced tumor growth. Lamins thus impede 3D migration but also promote survival against migration-induced stresses. | | | 24567359
 |
Aggrecan, link protein and tenascin-R are essential components of the perineuronal net to protect neurons against iron-induced oxidative stress.
Suttkus, A; Rohn, S; Weigel, S; Glöckner, P; Arendt, T; Morawski, M
Cell death & disease
5
e1119
2014
Afficher le résumé
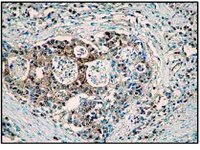
In Alzheimer's disease (AD), different types of neurons and different brain areas show differential patterns of vulnerability towards neurofibrillary degeneration, which provides the basis for a highly predictive profile of disease progression throughout the brain that now is widely accepted for neuropathological staging. In previous studies we could demonstrate that in AD cortical and subcortical neurons are constantly less frequently affected by neurofibrillary degeneration if they are enwrapped by a specialized form of the hyaluronan-based extracellular matrix (ECM), the so called 'perineuronal net' (PN). PNs are basically composed of large aggregating chondroitin sulphate proteoglycans connected to a hyaluronan backbone, stabilized by link proteins and cross-linked via tenascin-R (TN-R). Under experimental conditions in mice, PN-ensheathed neurons are better protected against iron-induced neurodegeneration than neurons without PN. Still, it remains unclear whether these neuroprotective effects are directly mediated by the PNs or are associated with some other mechanism in these neurons unrelated to PNs. To identify molecular components that essentially mediate the neuroprotective aspect on PN-ensheathed neurons, we comparatively analysed neuronal degeneration induced by a single injection of FeCl3 on four different mice knockout strains, each being deficient for a different component of PNs. Aggrecan, link protein and TN-R were identified to be essential for the neuroprotective properties of PN, whereas the contribution of brevican was negligible. Our findings indicate that the protection of PN-ensheathed neurons is directly mediated by the net structure and that both the high negative charge and the correct interaction of net components are essential for their neuroprotective function. | Immunohistochemistry | | 24625978
 |
The thrombospondin-1 receptor CD36 is an important mediator of ovarian angiogenesis and folliculogenesis.
Osz, K; Ross, M; Petrik, J
Reproductive biology and endocrinology : RB&E
12
21
2014
Afficher le résumé
Ovarian angiogenesis is a complex process that is regulated by a balance between pro- and anti-angiogenic factors. Physiological processes within the ovary, such as folliculogenesis, ovulation, and luteal formation are dependent upon adequate vascularization and anything that disrupts normal angiogenic processes may result in ovarian dysfunction, and possibly infertility. The objective of this study was to evaluate the role of the thrombospondin-1 (TSP-1) receptor CD36 in mediating ovarian angiogenesis and regulating ovarian function.The role of CD36 was evaluated in granulosa cells in vitro and ovarian morphology and protein expression were determined in wild type and CD36 null mice.In vitro, CD36 inhibition increased granulosa cell proliferation and decreased apoptosis. Granulosa cells in which CD36 was knocked down also exhibited an increase in expression of survival and angiogenic proteins. Ovaries from CD36 null mice were hypervascularized, with increased expression of pro-angiogenic vascular endothelial growth factor (VEGF) and its receptor VEGFR-2. Ovaries from CD36 null mice contained an increase in the numbers of pre-ovulatory follicles and decreased numbers of corpora lutea. CD36 null mice also had fewer number of offspring compared to wild type controls.The results from this study demonstrate that CD36 is integral to the regulation of ovarian angiogenesis by TSP-1 and the expression of these family members may be useful in the control of ovarian vascular disorders. | | | 24628875
 |
Electroacupuncture-like stimulation at Baihui and Dazhui acupoints exerts neuroprotective effects through activation of the brain-derived neurotrophic factor-mediated MEK1/2/ERK1/2/p90RSK/bad signaling pathway in mild transient focal cerebral ischemia in rats.
Cheng, CY; Lin, JG; Su, SY; Tang, NY; Kao, ST; Hsieh, CL
BMC complementary and alternative medicine
14
92
2014
Afficher le résumé
This study was designed to evaluate the effects of electroacupuncture-like stimulation at Baihui (GV20) and Dazhui (GV14) acupoints (EA at acupoints) following mild cerebral ischemia-reperfusion (I/R) injury. Furthermore, we investigated whether brain-derived neurotrophic factor (BDNF)-mediated activation of extracellular signal-regulated kinase (ERK)1/2 signaling pathway is involved in the neuroprotection induced by EA at acupoints.Rats were subjected to middle cerebral artery occlusion (MCAo) for 15 min followed by reperfusion for 3 d. EA at acupoints was applied 1 d postreperfusion then once daily for 2 consecutive days.Following the application of EA at acupoints, initiated 1 d postreperfusion, we observed significant reductions in the cerebral infarct area, neurological deficit scores, active caspase-3 protein expression, and apoptosis in the ischemic cortex after 3 d of reperfusion. We also observed markedly upregulated BDNF, phospho-Raf-1 (pRaf-1), phospho-MEK1/2 (pMEK1/2), phospho-ERK1/2 (pERK1/2), phospho-90 kDa ribosomal S6 kinase (pp90RSK), and phospho-Bad (pBad) expression, and restored neuronal nuclear antigen (NeuN) expression. Pretreatment with the MEK1/2 inhibitor U0126 abrogated the effects of EA at acupoints on cerebral infarct size, neurological deficits, active caspase-3 protein, and apoptosis in the ischemic cortex after 3 d of reperfusion. Pretreatment with U0126 also abrogated the effects of EA at acupoints on pMEK1/2, pERK1/2, pp90RSK, pBad, and NeuN expression, but did not influence BDNF and pRaf-1 expression.Overall, our study results indicated that EA at acupoints, initiated 1 d postreperfusion, upregulates BDNF expression to provide BDNF-mediated neuroprotection against caspase-3-dependent neuronal apoptosis through activation of the Raf-1/MEK1/2/ERK1/2/p90RSK/Bad signaling cascade after 3 d of reperfusion in mild MCAo. | Western Blotting | | 24606810
 |
Facile bench-top fabrication of enclosed circular microchannels provides 3D confined structure for growth of prostate epithelial cells.
Dolega, ME; Wagh, J; Gerbaud, S; Kermarrec, F; Alcaraz, JP; Martin, DK; Gidrol, X; Picollet-D'hahan, N
PloS one
9
e99416
2014
Afficher le résumé
We present a simple bench-top method to fabricate enclosed circular channels for biological experiments. Fabricating the channels takes less than 2 hours by using glass capillaries of various diameters (from 100 µm up to 400 µm) as a mould in PDMS. The inner surface of microchannels prepared in this way was coated with a thin membrane of either Matrigel or a layer-by-layer polyelectrolyte to control cellular adhesion. The microchannels were then used as scaffolds for 3D-confined epithelial cell culture. To show that our device can be used with several epithelial cell types from exocrine glandular tissues, we performed our biological studies on adherent epithelial prostate cells (non-malignant RWPE-1 and invasive PC3) and also on breast (non-malignant MCF10A) cells We observed that in static conditions cells adhere and proliferate to form a confluent layer in channels of 150 µm in diameter and larger, whereas cellular viability decreases with decreasing diameter of the channel. Matrigel and PSS (poly (sodium 4-styrenesulphonate)) promote cell adhesion, whereas the cell proliferation rate was reduced on the PAH (poly (allylamine hydrochloride))-terminated surface. Moreover infusing channels with a continuous flow did not induce any cellular detachment. Our system is designed to simply grow cells in a microchannel structure and could be easily fabricated in any biological laboratory. It offers opportunities to grow epithelial cells that support the formation of a light. This system could be eventually used, for example, to collect cellular secretions, or study cell responses to graduated hypoxia conditions, to chemicals (drugs, siRNA, …) and/or physiological shear stress. | | | 24945245
 |