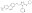531088 Sigma-AldrichVCP Inhibitor III, NMS-873 - CAS 1418013-75-8 - Calbiochem
A cell-permeable, non-ATP competitive, allosteric inhibitor of VCP ATPase (IC₅₀ = 20 nM with 1 mM ATP)
More>> A cell-permeable, non-ATP competitive, allosteric inhibitor of VCP ATPase (IC₅₀ = 20 nM with 1 mM ATP) Less<<Synonyms: ERAD Inhibitor III, NMS873, 3-(3-(Cyclopentylthio)-5-((2-methyl-4ʹ-(methylsulfonyl)biphenyl-4-yloxy)methyl)-4H-1,2,4-triazol-4-yl)pyridine, 3-(3-Cyclopentylsulfanyl-5-(4ʹ-methanesulfonyl-2-methyl-biphenyl-4-yloxymethyl)-[1,2,4]triazol-4-yl)-pyridine, Valosin-containing Protein Inhibitor III, p97 Inhibitor III
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| CAS # | Empirical Formula |
|---|---|
| 1418013-75-8 | C₂₇H₂₈N₄O₃S₂ |
| Description | |
|---|---|
| Overview | This product has been discontinued. A cell-permeable triazolylpyridine compound that potently inhibits VCP ATPase activity in a non-ATP-competitive manner (IC50/[ATP] = 20 nM/1 mM & 30 nM/60 µM; [wt hVCP] = 10 nM) via an allosteric mechanism where the inhibitor targets the lateral tunnel formed by the D1 & D2 domains of one VCP protomer together with the D1 from the adjacent VCP molecule in the hexamer, effectively locking the protomer in a rigid conformation and thereby preventing the release of bound ADP for successful propagation of interprotomer ATP hydrolysis cycle, while being ineffective against a panel of 53 kinases or the ATPase activities of 4 other AAA (ATPase Associated with diverse/various cellular Activities) ATPases (NSF, SPATAS, VPS4B, and cdc6) and Hsp90 (by <15% at 10 µM). In agreement with siRNA-mediated VCP knockdown, NMS-873 is shown to prevent VCP from extracting ubiquitinated client proteins, including cyclin E & Mcl-1, for proteasomal degradation, resulting in cellular UPR (unfold protein response) activation, autophagy induction, and eventual apoptosis in HCT-116 cultures in a dose- and time-dependent manner (0.5 to 5 µM; 8 to 72 h). Anti-cancer studies using a panel of 40 various hematological and solid tumor lines reveal wide-spread sensitivities to NMS-873-induced killings (IC50 from 80 nM to 2 µM), differing in degrees than those observed with the proteasome inhibitor Bortezomib (Cat. No. Cat. No. 504314). Exhibits high clearance and low bioavailability in in vivo studies. |
| Catalogue Number | 531088 |
| Brand Family | Calbiochem® |
| Synonyms | ERAD Inhibitor III, NMS873, 3-(3-(Cyclopentylthio)-5-((2-methyl-4ʹ-(methylsulfonyl)biphenyl-4-yloxy)methyl)-4H-1,2,4-triazol-4-yl)pyridine, 3-(3-Cyclopentylsulfanyl-5-(4ʹ-methanesulfonyl-2-methyl-biphenyl-4-yloxymethyl)-[1,2,4]triazol-4-yl)-pyridine, Valosin-containing Protein Inhibitor III, p97 Inhibitor III |
| References | |
|---|---|
| References | Magnaghi, P., et al. 2013. Nat. Chem. Biol. 9, 548. Polucci, P., et al. 2013. J. Med. Chem. 56, 437. |
| Product Information | |
|---|---|
| CAS number | 1418013-75-8 |
| Form | Light beige powder |
| Hill Formula | C₂₇H₂₈N₄O₃S₂ |
| Chemical formula | C₂₇H₂₈N₄O₃S₂ |
| Reversible | Y |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | VCP |
| Purity | ≥98% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 531088 | 0 |
Documentation
VCP Inhibitor III, NMS-873 - CAS 1418013-75-8 - Calbiochem SDS
| Title |
|---|
References
| Reference overview |
|---|
| Magnaghi, P., et al. 2013. Nat. Chem. Biol. 9, 548. Polucci, P., et al. 2013. J. Med. Chem. 56, 437. |
| Data Sheet | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Note that this data sheet is not lot-specific and is representative of the current specifications for this product. Please consult the vial label and the certificate of analysis for information on specific lots. Also note that shipping conditions may differ from storage conditions.
|