530487 Sigma-AldrichStemSelect® PD 0332991 - CAS 827022-32-2 - Calbiochem
A cell-permeable, brain permeant, potent, reversible, ATP competitive inhibitor of Cdk4 and Cdk6 (IC₅₀ = 11, 9, and 15 nM for Cdk4/D1, Cdk4/D3 and Cdk6/D2, respectively).
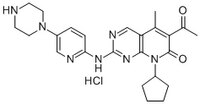
More>> A cell-permeable, brain permeant, potent, reversible, ATP competitive inhibitor of Cdk4 and Cdk6 (IC₅₀ = 11, 9, and 15 nM for Cdk4/D1, Cdk4/D3 and Cdk6/D2, respectively). Less<<Synonyms: 6-Acetyl-8-cyclopentyl-5-methyl-2-(5-(piperazin-1-yl)pyridin-2-ylamino)pyrido[2,3-d]pyrimidin-7(8H)-one, HCl, Cdk4/Cdk6 Inhibitor V, PD-0332991, HCl, PF-332991, HCl
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| CAS # | Empirical Formula |
|---|---|
| 827022-32-2 | C₂₄H₂₉N₇O₂ • HCl |
Products
| Catalogue Number | Packaging | Qty/Pack | |
|---|---|---|---|
| 5.30487.0001 | Glass bottle | 5 mg |
| Description | |
|---|---|
| Overview | A cell-permeable, orally available and brain permeant, non-toxic pyridopyrimidinone compound that acts as a potent, selective, reversible, ATP competitive inhibitor of Cdk4 and Cdk6 (IC50 = 11, 9, and 15 nM for Cdk4/D1, Cdk4/D3 and Cdk6/D2, respectively). Hence, it reduces retinoblastoma protein phosphorylation at Ser780/Ser795 (IC50 = 66 nM in MDA-435 cells) and arrests cell cycle at G1 phase. Acts as a cytostatic agent, but does induce apoptotic cell death when used alone. However, it potentiates the cytotoxicity of dexamethasone (Cat. No. 265005), bortezomib (Cat. No. 504314), and tamoxifen (Cat. No. 579000) in estrogen receptor (ER)-positive cell lines. Exhibits only a trivial inhibitory activity towards Cdk2/E2, Cdk2/A, Cdk1/B and Cdk5/p25 in a 36-kinase panel (IC50 > 10 µM). Improves endoderm differentiation of late G1-human embryonic stem cells expressing Smad2 or Smad3 (~ 750 nM) and further enhances endoderm differentiation into hepatic and pancreatic progenitor cells. Shown to regress the growth of human breast tumor xenografts in murine models (~150 mg/kg, p.o., daily). Please note that the molecular weight for this compound is batch-specific due to variable water content. |
| Catalogue Number | 530487 |
| Brand Family | Calbiochem® |
| Synonyms | 6-Acetyl-8-cyclopentyl-5-methyl-2-(5-(piperazin-1-yl)pyridin-2-ylamino)pyrido[2,3-d]pyrimidin-7(8H)-one, HCl, Cdk4/Cdk6 Inhibitor V, PD-0332991, HCl, PF-332991, HCl |
| Product Information | |
|---|---|
| CAS number | 827022-32-2 |
| Form | Yellow liquid |
| Hill Formula | C₂₄H₂₉N₇O₂ • HCl |
| Chemical formula | C₂₄H₂₉N₇O₂ • HCl |
| Reversible | Y |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | Cdk4 & Cdk6 |
| Purity | ≥97% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 5.30487.0001 | 04055977260823 |
Documentation
StemSelect® PD 0332991 - CAS 827022-32-2 - Calbiochem SDS
| Title |
|---|
References
| Reference overview |
|---|
| Pauklin, S. and Vallier, L., 2013. Cell 155, 135. Finn, R.S., et al. 2009. Breast Cancer Res. 11, R17. Menu, E., et al. 2008. Cancer Res. 68, 5519. Baughn, L.B., et al. 2006. Cancer Res. 66, 7661. Fry, D.W., et al. 2004. Mol. Cancer Ther. 3, 1427. |
| Data Sheet | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Note that this data sheet is not lot-specific and is representative of the current specifications for this product. Please consult the vial label and the certificate of analysis for information on specific lots. Also note that shipping conditions may differ from storage conditions.
|