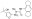538120 Sigma-AldrichNLRP3 Inhibitor, MCC950 - CAS 256373-96-3 - Calbiochem
A cell-potent, bioavailable NLRP3 inflammasome activation inhibitor. Effectively blocks caspase-1 & -11 activation, IL-1β processing and IL-1β & IL-6 secretion.
More>> A cell-potent, bioavailable NLRP3 inflammasome activation inhibitor. Effectively blocks caspase-1 & -11 activation, IL-1β processing and IL-1β & IL-6 secretion. Less<<Synonyms: N-(1,2,3,5,6,7-Hexahydro-s-indacen-4-ylcarbamoyl)-4-(2-hydroxy-2-propanyl)-2-furansulfonamide, Sodium, MCC-950, MCC950, Cytokine Release Inhibitory Drug 3, CRID 3, CP-456,773
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| CAS # | Empirical Formula |
|---|---|
| 256373-96-3 | C₂₀H₂₃N₂O₅S·Na |
Products
| Catalogue Number | Packaging | Qty/Pack | |
|---|---|---|---|
| 5.38120.0001 | Glass bottle | 10 mg |
| Product Information | |
|---|---|
| CAS number | 256373-96-3 |
| Form | White powder |
| Formulation | Supplied as a sodium salt. |
| Hill Formula | C₂₀H₂₃N₂O₅S·Na |
| Chemical formula | C₂₀H₂₃N₂O₅S·Na |
| Hygroscopic | Hygroscopic |
| Reversible | Y |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | NLRP3 |
| Purity | ≥97% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 5.38120.0001 | 04054839059308 |
Documentation
NLRP3 Inhibitor, MCC950 - CAS 256373-96-3 - Calbiochem SDS
| Title |
|---|
NLRP3 Inhibitor, MCC950 - CAS 256373-96-3 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 538120 |
References
| Reference overview |
|---|
| Coll, R.C., et al. 2015. Nat. Med. 21, 248. Coll, R.C. and O'Neill, L.A.J. 2011. PLoS ONE. 6, e29539. Laliberte, R.E., et al. 2003. J. Biol. Chem. 278, 16567. Perregaux, D.G., et al. 2001. J. Pharmacol. Exp. Ther. 299, 187. |