QIA39 Sigma-AldrichFragEL™ DNA Fragmentation Detection Kit, Fluorescent - TdT Enzyme
Recommended Products
-

ABN1644 Sigma-Aldrich Anti-C9orf72 (short form) -
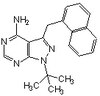
529581 Sigma-Aldrich PP1 Analog II, 1NM-PP1 - CAS 221244-14-0 - Calbiochem -

375240-25MG Sigma-Aldrich HNF4 Antagonist, BI6015 - CAS 93987-29-2 - Calbiochem -

1084350100 Supelco Titriplex® VI -

MABC14 Sigma-Aldrich Anti-VHL Antibody, clone 11E12 -

9710-OP Millipore OmniPur® Xylene Cyanol FF - CAS 2650-17-1 - Calbiochem -

1702450500 Supelco Vanadium standard solution -
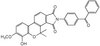
171262 Sigma-Aldrich AMPK Activator V, Ampkinone - CAS 1233082-79-5 - Calbiochem -
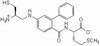
344550 Sigma-Aldrich FTI-276 - Calbiochem -

8211960250 Sigma-Aldrich Triphenylmethanol
Overview
Key Spec Table
| Detection Methods |
|---|
| Fluorescence |
Products
| Catalogue Number | Packaging | Qty/Pack | |
|---|---|---|---|
| QIA39-1EA | Fibre case | 1 ea |
| Description | |
|---|---|
| Overview | Allows for detection of apoptosis at the individual cell level. Apoptosis morphology is easily detected. Detection is by flow cytometry or fluorescence microscopy. The kit mounting media permits the observance of the total cell population. |
| Cat. No. QIA39 is a fluorescein-conjugated version of our widely referenced TdT Colorimetric FragEL™ DNA Fragmentation Detection Kit (Cat. No. QIA33) for non-isotopic labeling. Terminal deoxynucleotidyl transferase (TdT) binds to exposed 3′-OH ends of DNA fragments generated in response to apoptotic signals and catalyzes the addition of fluorescein-labeled and unlabeled deoxynucleotides. When excited, fluorescein generates an intense signal which can be detected by fluorescence microscopy or flow cytometry. The mounting media sustains the fluorescent signal from samples labeled on slides and, therefore, aids in the morphological evaluation and characterization of normal and apoptotic cells. Non-apoptotic cells can be visualized by a DAPI filter. | |
| Catalogue Number | QIA39 |
| Brand Family | Calbiochem® |
| Synonyms | TUNEL Assay |
| Materials Required but Not Delivered | • xylene • ethanol, 70, 80, 90, 100% • 4% formaldehyde in PBS • 10 mM Tris pH 8 • TBS (20 mM Tris pH 7.6, 140 mM NaCl) • 1 mM MgSO4 in TBS (optional, for use in generating positive control) • DNaseI (optional, for use in generating positive control) • distilled water • coplin jars, glass or plastic with slide holders • wash bottle or beaker for rinsing slides • humidified chamber • glass coverslips • fluorescence microscope or flow cytometer • 2-20 µl, 20-200 µl, and 200-1000 µl precision pipettors with disposable tips • microcentrifuge tubes • parafilm® • absorbent wipes • ice |
| Product Information | |
|---|---|
| Detection method | Fluorescence |
| Form | 50 Tests |
| Format | Flow cytometry or fluorescence microscopy |
| Kit contains | Proteinase K, Equilibration and Labeling Buffers, TdT Enzyme (Cat. No. PF060), Control Slides, Mounting Media (Cat. No. HC08), and a user protocol. |
| Quality Level | MQ100 |
| Applications | |
|---|---|
| Key Applications | Flow Cytometry |
| Biological Information | |
|---|---|
| Assay time | 2 h |
| Sample Type | Frozen or paraffin sections, fixed cell preparations or cell suspensions |
| Species Reactivity |
|
| Supplemental Information | |
|---|---|
| Kit contains | Proteinase K, Equilibration and Labeling Buffers, TdT Enzyme (Cat. No. PF060), Control Slides, Mounting Media (Cat. No. HC08), and a user protocol. |
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| QIA39-1EA | 07790788054045 |











