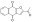
533851 Sigma-AldrichCancer Stemness Inhibitor, BBI608 - CAS 83280-65-3 - Calbiochem
A cell-permeable compound that diminishes gene expression and self-renewal in stemness high cancer cells. Blocks cancer relapse and metastasis.
More>> A cell-permeable compound that diminishes gene expression and self-renewal in stemness high cancer cells. Blocks cancer relapse and metastasis. Less<<Synonyms: 2-Acetyl-4H,9H-naphtho[2,3-b]furan-4,9-dione, 2-Acetylnaphtho[2,3-b]furan-4,9-dione, Spherogenesis Blocker, BBI-608
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| CAS # | Empirical Formula |
|---|---|
| 83280-65-3 | C₁₄H₈O₄ |
Products
| Catalogue Number | Packaging | Qty/Pack | |
|---|---|---|---|
| 5.33851.0001 | Glass bottle | 10 mg |
| References | |
|---|---|
| References | Li, Y., et al. 2015. Proc. Natl. Acad. Sci. USA 112, 1839. |
| Product Information | |
|---|---|
| CAS number | 83280-65-3 |
| Form | Brown solid |
| Hill Formula | C₁₄H₈O₄ |
| Chemical formula | C₁₄H₈O₄ |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | STAT3 |
| Primary Target IC<sub>50</sub> | 142 nM |
| Purity | ≥97% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 5.33851.0001 | 04055977286762 |
Documentation
Cancer Stemness Inhibitor, BBI608 - CAS 83280-65-3 - Calbiochem SDS
| Title |
|---|
References
| Reference overview |
|---|
| Li, Y., et al. 2015. Proc. Natl. Acad. Sci. USA 112, 1839. |