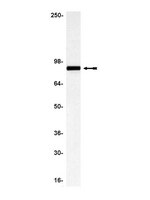
Polycystin-1 binds Par3/aPKC and controls convergent extension during renal tubular morphogenesis.
Castelli, M; Boca, M; Chiaravalli, M; Ramalingam, H; Rowe, I; Distefano, G; Carroll, T; Boletta, A
Nature communications
4
2658
2013
Show Abstract
Several organs, including the lungs and kidneys, are formed by epithelial tubes whose proper morphogenesis ensures correct function. This is best exemplified by the kidney, where defective establishment or maintenance of tubular diameter results in polycystic kidney disease, a common genetic disorder. Most polycystic kidney disease cases result from loss-of-function mutations in the PKD1 gene, encoding Polycystin-1, a large receptor of unknown function. Here we demonstrate that PC-1 has an essential role in the establishment of correct tubular diameter during nephron development. Polycystin-1 associates with Par3 favouring the assembly of a pro-polarizing Par3/aPKC complex and it regulates a programme of cell polarity important for oriented cell migration and for a convergent extension-like process during tubular morphogenesis. Par3 inactivation in the developing kidney results in defective convergent extension and tubular morphogenesis, and in renal cyst formation. Our data define Polycystin-1 as central to cell polarization and to epithelial tube morphogenesis and homeostasis. | Western Blotting, Immunofluorescence | 24153433
 |
Proteomics analysis of the ezrin interactome in B cells reveals a novel association with Myo18a?.
Ken Matsui,Neetha Parameswaran,Nayer Bagheri,Belinda Willard,Neetu Gupta
Journal of proteome research
10
2011
Show Abstract
The molecular regulation of recruitment and assembly of signalosomes near the B cell receptor (BCR) is poorly understood. We have previously demonstrated a role for the ERM family protein ezrin in regulating antigen-dependent lipid raft coalescence in B cells. In this study, we addressed the possibility that ezrin may collaborate with other adaptor proteins to regulate signalosome dynamics at the membrane. Using mass spectrometry-based proteomics analysis, we identified Myo18a? as a novel binding partner of ezrin. Myo18a? is an attractive candidate as it has several protein-protein interaction domains and an intrinsic motor activity. The expression of Myo18a? varied during B cell development in the bone marrow and in mature B cell subsets suggesting functional differences. Interestingly, BCR stimulation increased the association between ezrin and Myo18a?, and induced co-segregation of Myo18a? with the BCR and phosphotyrosine-containing proteins. Our data raise an intriguing possibility that the Myo18a?/ezrin complex may facilitate BCR-mediated signaling by recruiting signaling proteins that are in close proximity of the antigen receptor. Our study is not only significant with respect to understanding the molecular regulation of BCR signaling but also provides a broader basis for understanding the mechanism of action of ezrin in other cellular systems. | | 21751808
 |
Extranuclear signaling of mutated thyroid hormone receptors in promoting metastatic spread in thyroid carcinogenesis.
Changxue Lu,Sheue-Yann Cheng
Steroids
76
2011
Show Abstract
Thyroid hormone receptors (TRs) mediate the critical activities of the thyroid hormone (T3) in growth, development, and differentiation. Decreased expression and/or somatic mutations of TRs have been shown to be associated with several types of human cancers including liver, breast, lung, and thyroid. A direct demonstration that TRβ mutants could function as oncogenes is evidenced by the spontaneous development of follicular thyroid carcinoma similar to human cancer in a knockin mouse model harboring a mutated TRβ (denoted as PV; Thrb(PV/PV) mice). PV is a dominant negative mutation identified in a patient with resistance to thyroid hormone. Analysis of altered gene expression and molecular studies of thyroid carcinogenesis in Thrb(PV/PV) mice show that the oncogenic activity of PV is mediated by both nucleus-initiated transcription and extranuclear actions to alter gene expression and signaling transduction activity. This article focuses on recent findings of novel extranuclear actions of PV that affect signaling cascades and thereby the invasiveness, migration, and motility of thyroid tumor cells. These findings have led to identification of potential molecular targets for treatment of metastatic thyroid cancer. | | 21473875
 |
Beta-catenin is necessary to keep cells of ureteric bud/Wolffian duct epithelium in a precursor state.
Marose, TD; Merkel, CE; McMahon, AP; Carroll, TJ
Developmental biology
314
112-26
2008
Show Abstract
Differentiation is the process by which tissues/organs take on their final, physiologically functional form. This process is mediated in part by the silencing of embryonic genes and the activation of terminal, differentiation gene products. Mammalian kidney development is initiated when the Wolffian duct branches and invades the overlying metanephric mesenchyme. The newly formed epithelial bud, known as the ureteric bud, will continue to branch ultimately differentiating into the collecting duct system and ureter. Here, we show that Hoxb7-Cre mediated removal of beta-catenin from the mouse Wolffian duct epithelium leads to the premature expression of gene products normally associated with the differentiated kidney collecting duct system including the water channel protein, Aquaporin-3 and the tight junction protein isoform, ZO-1 alpha+. Mutant cells fail to maintain expression of some genes associated with embryonic development, including several mediators of branching morphogenesis, which subsequently leads to kidney aplasia or hypoplasia. Reciprocally, expression of a stabilized form of beta-catenin appears to block differentiation of the collecting ducts. All of these defects occur in the absence of any effects on the adherens junctions. These data indicate a role for beta-catenin in maintaining cells of the Wolffian ducts and the duct derived ureteric bud/collecting duct system in an undifferentiated or precursor state. Full Text Article | | 18177851
 |
Myc down-regulation as a mechanism to activate the Rb pathway in STAT5A-induced senescence.
Mallette, FA; Gaumont-Leclerc, MF; Huot, G; Ferbeyre, G
The Journal of biological chemistry
282
34938-44
2007
Show Abstract
Senescence is a general antiproliferative program that avoids the expansion of cells bearing oncogenic mutations. We found that constitutively active STAT5A (ca-STAT5A) can induce a p53- and Rb-dependent cellular senescence response. However, ca-STAT5A did not induce p21 and p16(INK4a), which are responsible for inhibiting cyclin-dependent protein kinases and engaging the Rb pathway during the senescence response to oncogenic ras. Intriguingly, ca-STAT5A led to a down-regulation of Myc and Myc targets, including CDK4, a negative regulator of Rb. The down-regulation of Myc was in part proteasome-dependent and correlated with its localization to promyelocytic leukemia bodies, which were found to be highly abundant during STAT5-induced senescence. Introduction of CDK4 or Myc bypassed STAT5A-induced senescence in cells in which p53 was also inactivated. These results uncover a novel mechanism to engage the Rb pathway in oncogene-induced senescence and indicate the existence of oncogene-specific pathways that regulate senescence. | | 17913706
 |