345834 Sigma-AldrichGenistein, Soybean - CAS 446-72-0 - Calbiochem
A cell-permeable, reversible, substrate competitive inhibitor of protein tyrosine kinases, including autophosphorylation of epidermal growth factor receptor kinase (IC₅₀ = 2.6 µM).
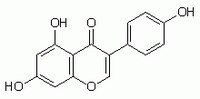
More>> A cell-permeable, reversible, substrate competitive inhibitor of protein tyrosine kinases, including autophosphorylation of epidermal growth factor receptor kinase (IC₅₀ = 2.6 µM). Less<<Synonyms: 4ʹ,5,7-Trihydroxyisoflavone
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| CAS # | Empirical Formula |
|---|---|
| 446-72-0 | C₁₅H₁₀O₅ |
Products
| Catalogue Number | Packaging | Qty/Pack | |
|---|---|---|---|
| 345834-20MGCN | 玻璃瓶 | 20 mg | |
| 345834-50MGCN | 塑膠安瓿;塑膠針藥瓶 | 50 mg |
| Product Information | |
|---|---|
| CAS number | 446-72-0 |
| ATP Competitive | N |
| Form | White solid |
| Hill Formula | C₁₅H₁₀O₅ |
| Chemical formula | C₁₅H₁₀O₅ |
| Reversible | Y |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | EGFR kinase |
| Primary Target IC<sub>50</sub> | 2.6 µM against autophosphorylation of epidermal growth factor receptor kinase |
| Purity | ≥95% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS | |
|---|---|
| RTECS | NR2392000 |
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 345834-20MGCN | 04055977214376 |
| 345834-50MGCN | 04055977214383 |
Documentation
Genistein, Soybean - CAS 446-72-0 - Calbiochem MSDS
| Title |
|---|
Genistein, Soybean - CAS 446-72-0 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 345834 |
References
| Reference overview |
|---|
| Constantinou, A., and Huberman, E. 1995. Proc. Soc. Exp. Biol. Med. 208, 109. Wei, H., et al. 1995. Proc. Soc. Exp. Biol. Med. 208, 124. Wei, H., et al. 1995. Carcinogenesis 17, 73. Kobayashi, S., et al. 1994. J. Biol. Chem. 269, 9011. Migita, K., et al. 1994. J. Immunol. 153, 3457. Spinozzi, F., et al. 1994. Leuk. Res. 18, 431. Dhar, A., et al. 1990. Mol. Pharmacol. 37, 519. Hill, T.D., et al. 1990. Science 248, 1660. Dean, N.M., et al. 1989. Biochem. Biophys. Res. Commun. 165, 795. Akiyama, T., et al. 1987. J. Biol. Chem. 262, 5592. |
Brochure
| Title |
|---|
| Caspases and other Apoptosis Related Tools Brochure |
Citations
| Title | |
|---|---|
|
|