344095 Sigma-AldrichFluvastatin, Sodium Salt - CAS 93957-55-2 - Calbiochem
A synthetic HMG-CoA reductase inhibitor (IC₅₀ = 40-100 nM for human liver microsomes) that acts as an anti-hypercholesterolemic agent.
More>> A synthetic HMG-CoA reductase inhibitor (IC₅₀ = 40-100 nM for human liver microsomes) that acts as an anti-hypercholesterolemic agent. Less<<Synonyms: (±)-(3Rʹ,5Sʹ,6E)-7-[3-(4-Fluorophenyl)-1-isopropylindol-2-yl]-3,5-dihydroxy-6-heptenoate, sodium
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| CAS # | Empirical Formula |
|---|---|
| 93957-55-2 | C₂₄H₂₅FNNaO₄ |
Products
| Catalogue Number | Packaging | Qty/Pack | |
|---|---|---|---|
| 344095-25MGCN | 塑膠安瓿;塑膠針藥瓶 | 25 mg |
| Description | |
|---|---|
| Overview | A synthetic HMG-CoA reductase inhibitor (IC50 = 40-100 nM for human liver microsomes) that acts as an anti-hypercholesterolemic agent. Decreases the basal MMP-1 levels in culture media of endothelial cells in a time- and dose-dependent manner. Inhibits the formation of TBA-reactive substances in Fe(II)-supported peroxidation of liposomes (IC50 = 12 µM). Also available as a 10 mM solution in H2O(Cat. No. 344096). |
| Catalogue Number | 344095 |
| Brand Family | Calbiochem® |
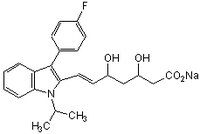
| Synonyms | (±)-(3Rʹ,5Sʹ,6E)-7-[3-(4-Fluorophenyl)-1-isopropylindol-2-yl]-3,5-dihydroxy-6-heptenoate, sodium |
| Product Information | |
|---|---|
| CAS number | 93957-55-2 |
| ATP Competitive | N |
| Form | Light yellow solid |
| Hill Formula | C₂₄H₂₅FNNaO₄ |
| Chemical formula | C₂₄H₂₅FNNaO₄ |
| Reversible | N |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | HMG-CoA reductase |
| Primary Target IC<sub>50</sub> | 40-100 nM against HMG-CoA reductase in human liver microsomes |
| Purity | ≥98% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | N |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS | |
|---|---|
| RTECS | MJ9675050 |
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 344095-25MGCN | 04055977215182 |
Documentation
Fluvastatin, Sodium Salt - CAS 93957-55-2 - Calbiochem MSDS
| Title |
|---|
Fluvastatin, Sodium Salt - CAS 93957-55-2 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 344095 |
References
| Reference overview |
|---|
| Yamamoto, A., et al. 2001. J. Pharm. Pharmacol. 53, 227. Dansette, P.M., et al. 2000. Exp. Toxicol. Pathol. 52, 145. Ikeda, U., et al. 2000. Hypertension 36, 325. Levy, R.I., et al. 1993. Circulation 87 (4 Suppl.), III45. Tse, F.L.S. et al. 1992. J. Clin. Pharmacol. 32, 630. Yuan, J., et al. 1991. Atherosclerosis 87, 147. |