124029 Sigma-AldrichAkt Inhibitor XII, Isozyme-Selective, Akti-2 - Calbiochem
Akt Inhibitor XII, Isozyme-Selective, Akti-2, is a cell-permeable, selective inhibitor of Akt2 (IC50 = 805 nM). The inhibition is PH domain-dependent and non-competitive with ATP.
More>> Akt Inhibitor XII, Isozyme-Selective, Akti-2, is a cell-permeable, selective inhibitor of Akt2 (IC50 = 805 nM). The inhibition is PH domain-dependent and non-competitive with ATP. Less<<Synonyms: 1-((3-(4-((4-(2,3-Dihydro-2-oxo-1H-benzimidazol-1-yl)-1-piperidinyl)methyl)phenyl)-2-phenyl-6-quinoxalinyl)carbonyl)-4-methyl-piperazine, 3HCl, 1-((2-(4-((4-(2,3-Dihydro-2-oxo-1H-benzimidazol-1-yl)-1-piperidinyl)methyl)phenyl)-3-phenyl-6-quinoxalinyl)carbonyl)-4-methyl-piperazine, 3HCl and
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| Empirical Formula |
|---|
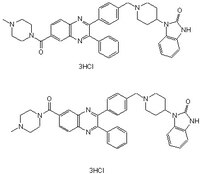
| C₃₉H₃₉N₇O₂ • 3HCl |
Products
| Catalogue Number | Packaging | Qty/Pack | |
|---|---|---|---|
| 124029-2MGCN | Glass bottle | 2 mg |
| Description | |
|---|---|
| Overview | A cell-permeable Akti-1/2- (Cat. No. 124018) derived allosteric inhibitor pair (1:1 regioisomeric mixture) with much improved aqueous solubility (10 mg/ml in a pH 7.4 saline) and Akt2 selectivity (IC50 = 805 nM for Akt2, >10 µM for Akt1/3, and >50 µM for PKA/PKC/SGK). The inhibition is PH domain-dependent and non-competitive with ATP. |
| Catalogue Number | 124029 |
| Brand Family | Calbiochem® |
| Synonyms | 1-((3-(4-((4-(2,3-Dihydro-2-oxo-1H-benzimidazol-1-yl)-1-piperidinyl)methyl)phenyl)-2-phenyl-6-quinoxalinyl)carbonyl)-4-methyl-piperazine, 3HCl, 1-((2-(4-((4-(2,3-Dihydro-2-oxo-1H-benzimidazol-1-yl)-1-piperidinyl)methyl)phenyl)-3-phenyl-6-quinoxalinyl)carbonyl)-4-methyl-piperazine, 3HCl and |
| References | |
|---|---|
| References | Zhao, Z., et al. 2008. Bioorg. Med. Chem. Lett. 18, 49. |
| Product Information | |
|---|---|
| Form | Yellow solid |
| Hill Formula | C₃₉H₃₉N₇O₂ • 3HCl |
| Chemical formula | C₃₉H₃₉N₇O₂ • 3HCl |
| Hygroscopic | Hygroscopic |
| Structure formula Image | |
| Quality Level | MQ100 |
| Biological Information | |
|---|---|
| Purity | ≥97% by HPLC (mixture of regioisomers) |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 124029-2MGCN | 04055977205763 |
Documentation
Akt Inhibitor XII, Isozyme-Selective, Akti-2 - Calbiochem SDS
| Title |
|---|
Akt Inhibitor XII, Isozyme-Selective, Akti-2 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 124029 |
References
| Reference overview |
|---|
| Zhao, Z., et al. 2008. Bioorg. Med. Chem. Lett. 18, 49. |
| Data Sheet | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Note that this data sheet is not lot-specific and is representative of the current specifications for this product. Please consult the vial label and the certificate of analysis for information on specific lots. Also note that shipping conditions may differ from storage conditions.
|