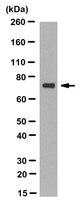
Molecular chaperone TRAP1 regulates a metabolic switch between mitochondrial respiration and aerobic glycolysis.
Yoshida, S; Tsutsumi, S; Muhlebach, G; Sourbier, C; Lee, MJ; Lee, S; Vartholomaiou, E; Tatokoro, M; Beebe, K; Miyajima, N; Mohney, RP; Chen, Y; Hasumi, H; Xu, W; Fukushima, H; Nakamura, K; Koga, F; Kihara, K; Trepel, J; Picard, D; Neckers, L
Proceedings of the National Academy of Sciences of the United States of America
110
E1604-12
2013
Show Abstract
TRAP1 (TNF receptor-associated protein), a member of the HSP90 chaperone family, is found predominantly in mitochondria. TRAP1 is broadly considered to be an anticancer molecular target. However, current inhibitors cannot distinguish between HSP90 and TRAP1, making their utility as probes of TRAP1-specific function questionable. Some cancers express less TRAP1 than do their normal tissue counterparts, suggesting that TRAP1 function in mitochondria of normal and transformed cells is more complex than previously appreciated. We have used TRAP1-null cells and transient TRAP1 silencing/overexpression to show that TRAP1 regulates a metabolic switch between oxidative phosphorylation and aerobic glycolysis in immortalized mouse fibroblasts and in human tumor cells. TRAP1-deficiency promotes an increase in mitochondrial respiration and fatty acid oxidation, and in cellular accumulation of tricarboxylic acid cycle intermediates, ATP and reactive oxygen species. At the same time, glucose metabolism is suppressed. TRAP1-deficient cells also display strikingly enhanced invasiveness. TRAP1 interaction with and regulation of mitochondrial c-Src provide a mechanistic basis for these phenotypes. Taken together with the observation that TRAP1 expression is inversely correlated with tumor grade in several cancers, these data suggest that, in some settings, this mitochondrial molecular chaperone may act as a tumor suppressor. | 23564345
 |
The hsp90-related protein TRAP1 is a mitochondrial protein with distinct functional properties.
Felts, SJ; Owen, BA; Nguyen, P; Trepel, J; Donner, DB; Toft, DO
The Journal of biological chemistry
275
3305-12
2000
Show Abstract
The hsp90 family of molecular chaperones was expanded recently due to the cloning of TRAP1 and hsp75 by yeast two-hybrid screens. Careful analysis of the human TRAP1 and hsp75 sequences revealed that they are identical, and we have cloned a similar protein from Drosophila. Immunofluorescence data show that human TRAP1 is localized to mitochondria. This mitochondrial localization is supported by the existence of mitochondrial localization sequences in the amino termini of both the human and Drosophila proteins. Due to the striking homology of TRAP1 to hsp90, we tested the ability of TRAP1 to function as an hsp90-like chaperone. TRAP1 did not form stable complexes with the classic hsp90 co-chaperones p23 and Hop (p60). Consistent with these observations, TRAP1 had no effect on the hsp90-dependent reconstitution of hormone binding to the progesterone receptor in vitro, nor could it substitute for hsp90 to promote maturation of the receptor to its hormone-binding state. However, TRAP1 is sufficiently conserved with hsp90 such that it bound ATP, and this binding was sensitive to the hsp90 inhibitor geldanamycin. In addition, TRAP1 exhibited ATPase activity that was inhibited by both geldanamycin and radicicol. Thus, TRAP1 has functions that are distinct from those of hsp90. | 10652318
 |