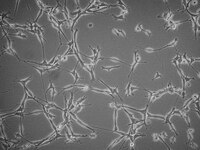
SCC246 Sigma-AldrichYUMMER1G.1F Mouse Cell Line
The characteristics of the YUMMER1.G1F cell line make it a valuable model for investigating sex-related differences in immune checkpoint inhibition and mechanisms of anti-tumor responses.
More>> The characteristics of the YUMMER1.G1F cell line make it a valuable model for investigating sex-related differences in immune checkpoint inhibition and mechanisms of anti-tumor responses. Less<<Recommended Products
Overview
| Replacement Information |
|---|
| References |
|---|
| Product Information | |
|---|---|
| Quality Level | MQ100 |
| Biological Information | |
|---|---|
| Cell Line Type |
|
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements | |
|---|---|
| Quality Assurance | • Each vial contains ≥ 1X106 viable cells. • Cells are tested negative for infectious diseases by a Mouse Essential CLEAR Panel by Charles River Animal Diagnostic Services. • Cells are verified to be of mouse origin and negative for inter-species contamination from rat, human, chinese hamster, Golden Syrian hamster, and Non-human Primate (NHP) as assessed by a Contamination Clear panel by Charles River Animal Diagnostic Services • Cells are negative for mycoplasma contamination. |
| Usage Statement |
|
| Storage and Shipping Information |
|---|
| Packaging Information | |
|---|---|
| Material Size | ≥1X10⁶ cells/vial |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| SCC246 | 04065266920123 |
Documentation
YUMMER1G.1F Mouse Cell Line MSDS
| Title |
|---|
Data Sheet
| Title |
|---|
| Data Sheet-SCC246 |
| Data Sheet-SCC246 |