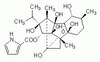
574590 Sigma-AldrichSuperoxide Anion Detection Kit, Chemiluminescent
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| Detection Methods |
|---|
| Luminescence |
| Applications |
|---|
| Biological Information | |
|---|---|
| Assay time | 1.5 h |
| Sample Type | Cell cultures |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information |
|---|
| Transport Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 574590 | 0 |
Documentation
Superoxide Anion Detection Kit, Chemiluminescent Certificates of Analysis
| Title | Lot Number |
|---|---|
| 574590 |
References
| Reference overview |
|---|
| Irami, K., et al. 1997. Science 275, 1649. Stohs, S.J. 1995. J. Basic Clin. Phys. Pharmacol. 6, 205. Tanaka, M., et al. 1994. J. Neurochem. 63, 266. Wang, J.F., et al. 1991. Biochem. J. 279, 311. Lomax, K.J., et al., 1989. Science 245, 409. Vilim, V., et al. 1989, Free Rad. Biol. Med. 6, 623. Kozumbo, W., et al. 1985. Chem.-Biol. Interact. 54, 199. Nakazawa, A., et al. 1985. Biochem. Pharmacol. 34, 481. Allred, C., et al. 1980. Biochem. Biophys. Acta 631, 380. |
Brochure
| Title |
|---|
| Kit SourceBook - 2nd Edition EURO |