Steritest™ NEO Devices for Antibiotics and Products with Antimicrobial Activity
Red canister base with specific drain design. Low binding PVDF Durapore® membrane. This optimizes the rinsing of products that inhibit microbial growth. Canisters material: SAN
Less<<
Related Resources
Recommended Products
Overview
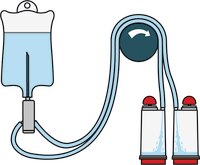
Liquids in Ampoules
TZHVAB210 & TZHVAB205 (double packed)
- Single needle for easy access to ampoules
- Separate vent needle
Liquids in Collapsible Bags
TZHVAB210 & TZHVAB205 (double packed)
- Single needle for easy access to collapsible bags
- Separate vent needle
Liquids in Large Vials
TZHVLV210 & TZHVLV205 (double packed)
- Vented double needle for large glass containers with septa
Liquids in Small Vials
TZHVSV210 & TZHVSV205 (double packed)
- Vented double needle for small vials with septa
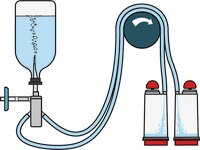
Soluble Powders in Vials
TZHVDV210 & TZHVDV205 (double packed)
- Double needles for small vials with septa
- Vented double needle
- Simultaneously dissolves/dilutes the sample in sterile diluent and transfers the resulting solution to canisters
Soluble Powders in Ampoules
TZHVDA210
- Double needles for small vials with septa
- Vented double needle
- Simultaneously dissolves/dilutes the sample in sterile diluent and transfers the resulting solution to canisters
Medical Devices and Collapsible Bags
TZHVMD210
- Three adapters provided; male Luer, female Luer or single needle allow connection to a variety of test devices
- Separate vent needle
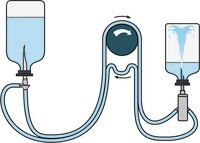
Powders and Superpotent Antibiotics
TZVC00010 + TZHVAB210 or TZHVAB205 (double packed)
- Tubing and needle assembly for antibiotics and products containing antimicrobial activity that require dilution or dissolution
- Aseptically connects the diluent or dissolution fluid to the product container for dilution
- Used for pooling superpotent antibiotics to reduce product membrane contact time when product is then filtered
- Contains vent with expansion chamber for optimized venting
- Diluted product subsequently filtered with Steritest™ NEO device (TZHVAB210)