530587 Sigma-AldrichProteasome Inhibitor XXIII, PI-1840 - Calbiochem
A cell-permeable, highly potent, selective, and non-covalent inhibitor of chymotrypsin-like (CT-L) activity of 20S proteasome (IC₅₀ = 27 nM).
More>> A cell-permeable, highly potent, selective, and non-covalent inhibitor of chymotrypsin-like (CT-L) activity of 20S proteasome (IC₅₀ = 27 nM). Less<<Synonyms: N-Isopropyl-2-(4-propylphenoxy)-N-((3-(pyridin-3-yl)-1,2,4-oxadiazol-5-yl)methyl)acetamide, PI1840
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| Empirical Formula |
|---|
| C₂₂H₂₆N₄O₃ |
| Description | |
|---|---|
| Overview | This product has been discontinued. A cell-permeable, bioavailable, non-toxic pyridinyl-oxadiazoloisopropylamide compound that acts as a highly potent, selective, and non-covalent inhibitor of chymotrypsin-like (CT-L) activity of 20S proteasome (IC50 = 27 nM). Does not affect tyrpsin-like and postglutamyl-peptide-hydrolysis-like activities of proteasome even at higher concentrations (IC50 >> 100 µM). Its action appears to be readily reversible. Displays over 120-fold greater selectivity for the constitutive proteasome over the immunoproteasome (IC50 = 18 nM vs. 2.17 µM). Induces the accumulation of p27, IκBα and Bax in MDA-MB-468 cells and reduces the viability of a broad spectrum of cancer cells and triggers apoptosis. Shown to increase sensitivity of HCT-116 cells to Nutlin (Cat. No. 444143) by about four- fold, but does not affect p53 -/- HCT-116 cells. Also shown to enhance sensitivity of LNCaP cells to BCl-2 antagonist, BH3-M6 by four-fold, but does not affect DU-145 cells that lack Bax. Suppresses the growth of MDA-MB-231 xenografts in nude mice (150 mg/kg, i.p., q.d.). |
| Catalogue Number | 530587 |
| Brand Family | Calbiochem® |
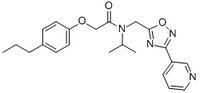
| Synonyms | N-Isopropyl-2-(4-propylphenoxy)-N-((3-(pyridin-3-yl)-1,2,4-oxadiazol-5-yl)methyl)acetamide, PI1840 |
| References | |
|---|---|
| References | Kazi, A., et al. 2014. J. Biol. Chem. 289, 11906. Ozcan, S., et al. 2013. J. Med. Chem. 56, 3783. |
| Product Information | |
|---|---|
| Form | White solid |
| Hill Formula | C₂₂H₂₆N₄O₃ |
| Chemical formula | C₂₂H₂₆N₄O₃ |
| Reversible | Y |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | chymotrypsin-like activity of 20S proteasome |
| Primary Target IC<sub>50</sub> | 27 nM for chymotrypsin-like (CT-L) activity of 20S proteasome |
| Purity | ≥98% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 530587 | 0 |
Documentation
Proteasome Inhibitor XXIII, PI-1840 - Calbiochem MSDS
| Title |
|---|
References
| Reference overview |
|---|
| Kazi, A., et al. 2014. J. Biol. Chem. 289, 11906. Ozcan, S., et al. 2013. J. Med. Chem. 56, 3783. |
| Data Sheet | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Note that this data sheet is not lot-specific and is representative of the current specifications for this product. Please consult the vial label and the certificate of analysis for information on specific lots. Also note that shipping conditions may differ from storage conditions.
|