495604 Sigma-AldrichOkadaic Acid, Prorocentrum sp. - CAS 78111-17-8 - Calbiochem
Okadaic Acid, CAS 78111-17-8, is a highly potent inhibitor of protein phosphatase 1 (IC₅₀ = 10-15 nM) and 2A (IC₅₀ = 0.1 nM).
More>> Okadaic Acid, CAS 78111-17-8, is a highly potent inhibitor of protein phosphatase 1 (IC₅₀ = 10-15 nM) and 2A (IC₅₀ = 0.1 nM). Less<<Synonyms: OA
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| CAS # | Empirical Formula |
|---|---|
| 78111-17-8 | C₄₄H₆₈O₁₃ |
| Description | |
|---|---|
| Overview | An ionophore-like polyether derivative of a C38 fatty acid compound that has tumor promoting properties. Potent inhibitor of protein phosphatase 1 (IC50 = 10-15 nM) and protein phosphatase 2A (IC50 = 0.1 nM). Does not affect the activity of tyrosine phosphatases, alkaline phosphatases, or acid phosphatases. Useful for the study of protein phosphatases in cell extracts as well as in intact cells. Induces apoptosis in human breast carcinoma cells (MB-231 and MCF7) and in myeloid cells but inhibits glucocorticoid-induced apoptosis in T cell hybridomas. Has marked contractile effects on smooth muscle and heart muscle. Implicated as causative agent of diarrhetic shellfish poisoning. A 250 µM (25 µg/124 µl) solution of Okadaic Acid, (Cat. No. 495609) in DMSO is also available. |
| Catalogue Number | 495604 |
| Brand Family | Calbiochem® |
| Synonyms | OA |
| Product Information | |
|---|---|
| CAS number | 78111-17-8 |
| ATP Competitive | N |
| Form | Colorless glassy solid |
| Hill Formula | C₄₄H₆₈O₁₃ |
| Chemical formula | C₄₄H₆₈O₁₃ |
| Reversible | N |
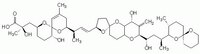
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | Protein phosphatase |
| Primary Target IC<sub>50</sub> | 10-15 nM and 0.1 nM against protein phosphatase 1 and protein phosphatase 2A, respectively |
| Purity | ≥95% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | N |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS | |
|---|---|
| RTECS | AA8227800 |
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 495604 | 0 |
Documentation
Okadaic Acid, Prorocentrum sp. - CAS 78111-17-8 - Calbiochem MSDS
| Title |
|---|
Okadaic Acid, Prorocentrum sp. - CAS 78111-17-8 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 495604 |
References
| Reference overview |
|---|
| Gjertsen, B.T., et al. 1994. J. Cell Sci. 107, 3363. Kiguchi, K., et al. 1994. Cell Growth Differentiation 5, 995. Ohaka, Y., et al. 1993. Biochem. Biophys. Res. Commun. 197, 916. Gopalakrishna, R., et al. 1992. Biochem. Biophys. Res. Commun. 189, 950. Kreienbuhl, P., et al. 1992. Blood 80, 2911. Nomura, M., et al. 1992. Biochemistry 31, 11915. Song, Q., et al. 1992. J. Cell Physiol. 153, 550. Tada, Y., et al. 1992. Immunopharmacol. 24, 17. Cohen, P., et al. 1990. Trends Biochem. Sci. 15, 98. Cohen, P. 1989. Annu. Rev. Biochem. 58, 453. Cohen, P., and Cohen, P.T. 1989. J. Biol. Chem. 264, 21435. Haystead, T.A., et al. 1989. Nature 337, 78. |
Brochure
| Title |
|---|
| Caspases and other Apoptosis Related Tools Brochure |
| Protein Phosphatases Technical Bulletin |
Citations
| Title | |
|---|---|
|
|