488004 Sigma-AldrichNodinitib-1 - Calbiochem
Synonyms: CID-1088438, 1-((4-Methylphenyl)sulfonyl)-1H-benzimidazol-2-amine, ML130, 1-(Toluene-4-sulfonyl)-1H-benzimidazol-2-ylamine, NOD1 Signaling Inhibitor, Nucleotide-binding Oligomerization Domain 1 Signaling Inhibitor
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| Empirical Formula |
|---|
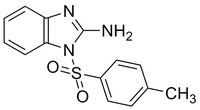
| C₁₄H₁₃N₃O₂S |
| References | |
|---|---|
| References | Khan, P.M., et al. 2011. ACS Med. Chem. Lett. 2, 780. Correa, R.G., et al. 2011. Chem. Biol. 18, 825. |
| Product Information | |
|---|---|
| Form | White powder |
| Hill Formula | C₁₄H₁₃N₃O₂S |
| Chemical formula | C₁₄H₁₃N₃O₂S |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Purity | ≥99% by HPLC |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 488004 | 0 |
Documentation
Nodinitib-1 - Calbiochem MSDS
| Title |
|---|
Nodinitib-1 - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 488004 |
References
| Reference overview |
|---|
| Khan, P.M., et al. 2011. ACS Med. Chem. Lett. 2, 780. Correa, R.G., et al. 2011. Chem. Biol. 18, 825. |