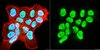
Adenovirus E4-ORF3-dependent relocalization of TIF1α and TIF1γ relies on access to the Coiled-Coil motif.
Vink, Elizabeth I, et al.
Virology, 422: 317-25 (2012)
2011
Show Abstract
The adenovirus E4-ORF3 protein promotes viral replication by relocalizing cellular proteins into nuclear track structures, interfering with potential anti-viral activities. E4-ORF3 targets transcriptional intermediary factor 1 alpha (TIF1α), but not homologous TIF1β. Here, we introduce TIF1γ as a novel E4-ORF3-interacting partner. E4-ORF3 relocalizes endogenous TIF1γ in virus-infected cells in vivo and binds to TIF1γ in vitro. We used the homologous nature, yet differing binding capabilities, of these proteins to study how E4-ORF3 targets proteins for track localization. We mapped the ability of E4-ORF3 to interact with specific TIF1 subdomains, demonstrating that E4-ORF3 interacts with the Coiled-Coil domains of TIF1α, TIF1β, and TIF1γ, and that the C-terminal half of TIF1β interferes with this interaction. The results of E4-ORF3-directed TIF1 protein relocalization assays performed in vivo were verified using coimmunoprecipitation assays in vitro. These results suggest that E4-ORF3 targets proteins for relocalization through a loosely homologous sequence dependent on accessibility. | 22123502
 |
Biochemical and functional properties of distinct nicotinic acetylcholine receptors in the superior cervical ganglion of mice with targeted deletions of nAChR subunit genes.
David, Reinhard, et al.
Eur. J. Neurosci., 31: 978-93 (2010)
2009
Show Abstract
Nicotinic acetylcholine receptors (nAChRs) mediate fast synaptic transmission in ganglia of the autonomic nervous system. Here, we determined the subunit composition of hetero-pentameric nAChRs in the mouse superior cervical ganglion (SCG), the function of distinct receptors (obtained by deletions of nAChR subunit genes) and mechanisms at the level of nAChRs that might compensate for the loss of subunits. As shown by immunoprecipitation and Western blots, wild-type (WT) mice expressed: alpha 3 beta 4 (55%), alpha 3 beta 4 alpha 5 (24%) and alpha 3 beta 4 beta 2 (21%) nAChRs. nAChRs in beta 4 knockout (KO) mice were reduced to < 15% of controls and no longer contained the alpha 5 subunit. Compound action potentials, recorded from the postganglionic (internal carotid) nerve and induced by preganglionic nerve stimulation, did not differ between alpha 5 beta 4 KO and WT mice, suggesting that the reduced number of receptors in the KO mice did not impair transganglionic transmission. Deletions of alpha 5 or beta2 did not affect the overall number of receptors and we found no evidence that the two subunits substitute for each other. In addition, dual KOs allowed us to study the functional properties of distinct alpha 3 beta4 and alpha 3 beta 2 receptors that have previously only been investigated in heterologous expression systems. The two receptors strikingly differed in the decay of macroscopic currents, the efficacy of cytisine, and their responses to the alpha-conotoxins AuIB and MII. Our data, based on biochemical and functional experiments and several mouse KO models, clarify and significantly extend previous observations on the function of nAChRs in heterologous systems and the SCG. | 20377613
 |
Novel irreversible epidermal growth factor receptor inhibitors by chemical modulation of the cysteine-trap portion.
Carmi, Caterina, et al.
J. Med. Chem., 53: 2038-50 (2010)
2009
Show Abstract
Irreversible EGFR inhibitors can circumvent acquired resistance to first-generation reversible, ATP-competitive inhibitors in the treatment of non-small-cell lung cancer. They contain both a driver group, which assures target recognition, and a warhead, generally an acrylamide or propargylamide fragment that binds covalently to Cys797 within the kinase domain of EGFR. We performed a systematic exploration of the role for the warhead group, introducing different cysteine-trapping fragments at position 6 of a traditional 4-anilinoquinazoline scaffold. We found that different reactive groups, including epoxyamides (compounds 3-6) and phenoxyacetamides (compounds 7-9), were able to irreversibly inhibit EGFR. In particular, at significant lower concentrations than gefitinib (1), (2R,3R)-N-(4-(3-bromoanilino)quinazolin-6-yl)-3-(piperidin-1-ylmethyl)oxirane-2-carboxamide (6) inhibited EGFR autophosphorylation and downstream signaling pathways, suppressed proliferation, and induced apoptosis in gefitinib-resistant NSCLC H1975 cells, harboring the T790M mutation in EGFR. | 20151670
 |
Casein kinase 1alpha governs antigen-receptor-induced NF-kappaB activation and human lymphoma cell survival.
Bidère, Nicolas, et al.
Nature, 458: 92-6 (2009)
2009
Show Abstract
The transcription factor NF-kappaB is required for lymphocyte activation and proliferation as well as the survival of certain lymphoma types. Antigen receptor stimulation assembles an NF-kappaB activating platform containing the scaffold protein CARMA1 (also called CARD11), the adaptor BCL10 and the paracaspase MALT1 (the CBM complex), linked to the inhibitor of NF-kappaB kinase complex, but signal transduction is not fully understood. We conducted parallel screens involving a mass spectrometry analysis of CARMA1 binding partners and an RNA interference screen for growth inhibition of the CBM-dependent 'activated B-cell-like' (ABC) subtype of diffuse large B-cell lymphoma (DLBCL). Here we report that both screens identified casein kinase 1alpha (CK1alpha) as a bifunctional regulator of NF-kappaB. CK1alpha dynamically associates with the CBM complex on T-cell-receptor (TCR) engagement to participate in cytokine production and lymphocyte proliferation. However, CK1alpha kinase activity has a contrasting role by subsequently promoting the phosphorylation and inactivation of CARMA1. CK1alpha has thus a dual 'gating' function which first promotes and then terminates receptor-induced NF-kappaB. ABC DLBCL cells required CK1alpha for constitutive NF-kappaB activity, indicating that CK1alpha functions as a conditionally essential malignancy gene-a member of a new class of potential cancer therapeutic targets. | 19118383
 |