533060 Sigma-AldrichHMGB Inhibitor, Inflachromene - Calbiochem
An anti-inflamatory agent that directly binds to HMGB proteins and diminishes their cytoplasmic accumulation in microglial cells. Blocks LPS-induced nitrite release.
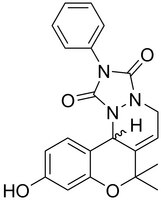
More>> An anti-inflamatory agent that directly binds to HMGB proteins and diminishes their cytoplasmic accumulation in microglial cells. Blocks LPS-induced nitrite release. Less<<Synonyms: Neuroinflammatory Inhibitor, Inflachromene, HMGB2 Inhibitor, Inflachromene, 10-Hydroxy-7,7-dimethyl-2-phenyl-7,12b-dihydrochromeno[4,3-c][1,2,4]triazolo[1,2-a]pyridazine-1,3(2H,5H)-dione
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| Empirical Formula |
|---|
| C₂₁H₁₉N₃O₄ |
| References | |
|---|---|
| References | Lee, S., et al. 2014. Nat. Chem. Biol. 10, in press. |
| Product Information | |
|---|---|
| Form | Off-white solid |
| Hill Formula | C₂₁H₁₉N₃O₄ |
| Chemical formula | C₂₁H₁₉N₃O₄ |
| Reversible | Y |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | HMGB2 |
| Purity | ≥98% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 533060 | 0 |
Documentation
HMGB Inhibitor, Inflachromene - Calbiochem MSDS
| Title |
|---|
References
| Reference overview |
|---|
| Lee, S., et al. 2014. Nat. Chem. Biol. 10, in press. |