362330 Sigma-AldrichGAG Antagonist, Surfen - Calbiochem
The GAG Antagonist, Surfen controls the biological activity of GAG. This small molecule/inhibitor is primarily used for Activators/Inducers applications.
More>> The GAG Antagonist, Surfen controls the biological activity of GAG. This small molecule/inhibitor is primarily used for Activators/Inducers applications. Less<<Synonyms: Gβγ Activator, 12155, NSC12155, bis-2-Methyl-4-amino-quinolyl-6-carbamide, diHCl, trihydrate, Glycosaminoglycans Antagonist
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| Empirical Formula |
|---|
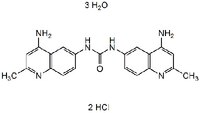
| C₂₁H₂₀N₆O • 2HCl • 3H₂O |
| Description | |
|---|---|
| Overview | A symmetrical quinolyl-urea compound that binds GAGs (glycosaminoglycans) via electrostatic interaction with the negatively charged sulfate and carboxyl moieties present in heparan sulfate (HS), heparin, and dermatan sulfate, resulting in effective blockage of GAGs interactions with their protein binding partners. Surfen is shown to effectively neutralize HS- and heparin-mediated thrombin inhibition of Factor Xa activity as well as heparin's anti-clotting activity. Also reported to inhibit FGF2-induced Erk phosphorylation and tubulation in murine lung endothelial cultures (IC50 ~5 µM), fibronectin HS-binding domain-dependent CHO cell adhesion (IC50 = 3 µM), and HSV-1 infection of glucosaminyl 3-O-sulfotransferase-3A-expressing CHO cells (complete inhibition at 5 µM). Surfen analogs with improved potency may serve as promising candidates as less toxic alternatives to Protamine (Cat. No. 539122) in clinical applications.Reported to directly bind Gβγ subunit in a reversible manner, displace Gα-GDP from Gβγ, and acutely activate Gβγ signaling without Gα activation. |
| Catalogue Number | 362330 |
| Brand Family | Calbiochem® |
| Synonyms | Gβγ Activator, 12155, NSC12155, bis-2-Methyl-4-amino-quinolyl-6-carbamide, diHCl, trihydrate, Glycosaminoglycans Antagonist |
| References | |
|---|---|
| References | Surve, C.R., et al. 2016. Sci. Signal. 9, ra22. Schuksz, M., et al. 2008. Proc. Natl. Acad. Sci. USA 105, 13074. Hunter, D.T. Jr., and Hill, J.M. 1961. Nature 191, 1378. |
| Product Information | |
|---|---|
| Form | White solid |
| Hill Formula | C₂₁H₂₀N₆O • 2HCl • 3H₂O |
| Chemical formula | C₂₁H₂₀N₆O • 2HCl • 3H₂O |
| Hygroscopic | Hygroscopic |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Purity | ≥98% by HPLC |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 362330 | 0 |
Documentation
GAG Antagonist, Surfen - Calbiochem MSDS
| Title |
|---|
GAG Antagonist, Surfen - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 362330 |
References
| Reference overview |
|---|
| Surve, C.R., et al. 2016. Sci. Signal. 9, ra22. Schuksz, M., et al. 2008. Proc. Natl. Acad. Sci. USA 105, 13074. Hunter, D.T. Jr., and Hill, J.M. 1961. Nature 191, 1378. |