508320 Sigma-AldrichEzh2 Inhibitor III, SAH-EZH2 - Calbiochem
A helical 27-mer peptide with E54Q modification that directly binds to EDD (Kd = 320 nM) and disrupts protein-protein interaction of EED with EZH2 and EZH1.
More>> A helical 27-mer peptide with E54Q modification that directly binds to EDD (Kd = 320 nM) and disrupts protein-protein interaction of EED with EZH2 and EZH1. Less<<Synonyms: H3K27 Methylation Inhibitor, SAH-EZH2, EED-EZH2 Protein-Protein Interaction Inhibitor, SAH-EZH2, Enhancer of Zested Homolog 2 Inhibitor III, HMTase Inhibitor XIV
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| Empirical Formula |
|---|
| C₁₅₅H₂₅₆N₄₈O₄₀·6CF₃CO₂H·XXH₂O |
Products
| Catalogue Number | Packaging | Qty/Pack | |
|---|---|---|---|
| 5.08320.0001 | Sklená flaša | 2 mg |
| References | |
|---|---|
| References | Kim, W., et al. 2013. Nat. Chem. Biol. 9, 643. |
| Product Information | |
|---|---|
| Form | White powder |
| Formulation | Supplied as a trifluoroacetate salt. |
| Hill Formula | C₁₅₅H₂₅₆N₄₈O₄₀·6CF₃CO₂H·XXH₂O |
| Chemical formula | C₁₅₅H₂₅₆N₄₈O₄₀·6CF₃CO₂H·XXH₂O |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | EED (embryonic ectoderm development) - EZH2 |
| Primary Target K<sub>i</sub> | 320 nM |
| Purity | ≥95% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
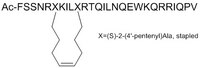
| Peptide Sequence | Ac-FSSNRXKILXRTQILNQEWKQRRIQPV (X=(S)-2-(4ʹ-pentenyl)Ala, stapled) |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 5.08320.0001 | 04055977261882 |
Documentation
Ezh2 Inhibitor III, SAH-EZH2 - Calbiochem MSDS
| Title |
|---|
References
| Reference overview |
|---|
| Kim, W., et al. 2013. Nat. Chem. Biol. 9, 643. |