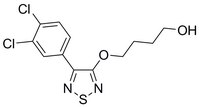
533980 Sigma-AldrichEMT Pathways Inhibitor, C19
A cell-permeable, bioavailable, potent and reversible multi EMT (Hippo, Wnt and TGFβ) pathway inhibitor. Blocks cancer cell migration and inhibits melanoma tumor growth.
More>> A cell-permeable, bioavailable, potent and reversible multi EMT (Hippo, Wnt and TGFβ) pathway inhibitor. Blocks cancer cell migration and inhibits melanoma tumor growth. Less<<Synonyms: 4-(4-(3,4-Dichlorophenyl)-1,2,5-thiadiazol-3-yloxy)butan-1-ol, Epithelial-Mesenchymal Transition (EMT) Pathways Inhibitor, AMPK Signaling Activator XV; Hippo Pathway Inhibitor, Wnt Pathway Inhibitor XXIV
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| Empirical Formula |
|---|
| C₁₂H₁₂Cl₂N₂O₂S |
| Description | |
|---|---|
| Overview | A cell-permeable, bioavailable, thiadiazolyloxybutanol compound that acts as a potent and reversible inhibitor of multiple pathways involved in epithelial-mesenchymal transition (EMT; IC50 < 2 µM for Hippo signaling in HEK293 cells transfected with TEAD luciferase reporter system 8XGTIIC). Also shown to be an effective inhibitor of Wnt and TGF-β signaling pathways. Induces GSK-3β-mediated degradation of TAZ by activating Hippo kinases Mst1 and Lats1. Shown to be about 10-fold more potent than AICAR (Cat. no. 123040) in inducing phosphorylation (Thr172) and activation of AMPK leading to autophagy. However, it does not appear to induce apoptosis in cells. Shown to increase degradation of nuclear b-catenin and increase the level of cytosolic β-catenin in multiple cancer cell lines (WM266, SW480, and MCF7) and suppresses the expression of various genes associated with EMT (Zeb1, Snail, Hes, Myc and Cyclin D). Inhibits the growth of WM266 melanoma tµMor xenografts in mice (20 mg/kg, i.p., X 3 in 30 days) once in 3 days for 30 days) without affecting their body weights. Please note that the molecular weight for this compound is batch-specific due to variable water content. Please refer to the vial label or the certificate of analysis for the batch-specific molecular weight. The molecular weight provided represents the baseline molecular weight without water. |
| Catalogue Number | 533980 |
| Brand Family | Calbiochem® |
| Synonyms | 4-(4-(3,4-Dichlorophenyl)-1,2,5-thiadiazol-3-yloxy)butan-1-ol, Epithelial-Mesenchymal Transition (EMT) Pathways Inhibitor, AMPK Signaling Activator XV; Hippo Pathway Inhibitor, Wnt Pathway Inhibitor XXIV |
| References | |
|---|---|
| References | Basu, D., et al. 2014. Mol. Cancer Ther. 13, 1457. |
| Product Information | |
|---|---|
| Form | Off-white solid |
| Hill Formula | C₁₂H₁₂Cl₂N₂O₂S |
| Chemical formula | C₁₂H₁₂Cl₂N₂O₂S |
| Reversible | Y |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | multiple pathways involved in epithelial-mesenchymal transition |
| Purity | ≥98% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 533980 | 0 |
Documentation
EMT Pathways Inhibitor, C19 MSDS
| Title |
|---|
References
| Reference overview |
|---|
| Basu, D., et al. 2014. Mol. Cancer Ther. 13, 1457. |
| Data Sheet | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Note that this data sheet is not lot-specific and is representative of the current specifications for this product. Please consult the vial label and the certificate of analysis for information on specific lots. Also note that shipping conditions may differ from storage conditions.
|