219385 Sigma-AldrichCathepsin B Inhibitor II - Calbiochem
The Cathepsin B Inhibitor II controls the biological activity of Cathepsin B. This small molecule/inhibitor is primarily used for Protease Inhibitors applications.
More>> The Cathepsin B Inhibitor II controls the biological activity of Cathepsin B. This small molecule/inhibitor is primarily used for Protease Inhibitors applications. Less<<Synonyms: Ac-LVK-CHO
Recommended Products
Overview
| Replacement Information |
|---|
Key Spec Table
| Empirical Formula |
|---|
| C₁₉H₃₆N₄O₄ |
Products
| Catalogue Number | Packaging | Qty/Pack | |
|---|---|---|---|
| 219385-1MG | Plastová ampulka | 1 mg |
| Description | |
|---|---|
| Overview | A more active lysinal analog of Leupeptin (Cat. No. 108975). A more potent inhibitor of cathepsin B (IC50 = 4 nM) compared to leupeptin (IC50 = 310 nM). |
| Note: this peptide forms cyclic isomers. | |
| Catalogue Number | 219385 |
| Brand Family | Calbiochem® |
| Synonyms | Ac-LVK-CHO |
| References | |
|---|---|
| References | McConnell, R.M., et al. 1993. J. Med. Chem. 36, 1084. |
| Product Information | |
|---|---|
| ATP Competitive | N |
| Form | White solid |
| Hill Formula | C₁₉H₃₆N₄O₄ |
| Chemical formula | C₁₉H₃₆N₄O₄ |
| Reversible | N |
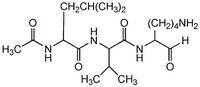
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | cathepsin B |
| Primary Target IC<sub>50</sub> | 4 nM against cathepsin B |
| Purity | ≥95% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | N |
| Peptide Sequence | Ac-Leu-Val-lysinal |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Catalogue Number | GTIN |
| 219385-1MG | 04055977201666 |
Documentation
Cathepsin B Inhibitor II - Calbiochem MSDS
| Title |
|---|
Cathepsin B Inhibitor II - Calbiochem Certificates of Analysis
| Title | Lot Number |
|---|---|
| 219385 |
References
| Reference overview |
|---|
| McConnell, R.M., et al. 1993. J. Med. Chem. 36, 1084. |
Brochure
| Title |
|---|
| Cathepsins and Related Products Technical Bulletin |
Citations
| Title | |
|---|---|
|
|
| Data Sheet | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Note that this data sheet is not lot-specific and is representative of the current specifications for this product. Please consult the vial label and the certificate of analysis for information on specific lots. Also note that shipping conditions may differ from storage conditions.
|