BrdU Antibodies, Kits, and Nucleotides
Recommended Products
-

1057391000 Supelco Molekulové sito 0,4 nm -

TMTP02500 Millipore Isopore Membrane Filter -

04-823 Sigma-Aldrich Anti-phospho-GluR1 (Ser831) Antibody, clone N453, rabbit monoclonal -

ZRDSVP3UK Milli-Q ZRDSVP3UK -

152610 Supelco TLC Explorer -
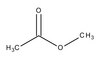
809711 Sigma-Aldrich Metylacetát -

1430071000 Supelco Hydrochloric acid solution -

459620-25UG Sigma-Aldrich Okadaic Acid, Sodium Salt - Calbiochem -

ZDELSPA06 ZDELSPA06 -

AB2253 Sigma-Aldrich Anti-Doublecortin Antibody
Overview
Bromodeoxyuridine (BrdU) is a thymidine analog and is specifically incorporated into DNA during DNA synthesis. Anti-bromodeoxyuridine antibody is used to identify cells that have incorporated BrdU. Anti-bromodeoxyuridine staining with flow cytometric analysis allows multiple parameters to be evaluated simultaneously. Anti-bromodeoxyuridine antibody has been used for identifying proliferating cells in blood, tissues , tumors, as well as for determining plasma cell labeling indices.
BrdU Labeling & ICC Protocol
Adherent or non-adherent cells can be labeled. Usually the labeling is done for a short period, such as two hours as shown in this example. However one can label overnight or even days depending upon the experiment.
Immunocytochemistry Protocol
1. Grow HUVEC cells in T flasks in traditional DMEM with 10% FBS.
2. Dissociate cells using trypsin/EDTA solution, and transfer to shell vials with 12 mm coverslips in 1 mL of appropriate media. (Alternatively polylysine-coated coverslips [0.05–0.1% in PBS,overnight using 150–300K mol. wt.] in 24 well plates or chamber-slides.)
3. Incubate cells at 37°C for 2 hours to allow for recovery.
4. Add BrdU* to each shell vial to a final concentration of 10 µM and incubate at 37°C for 2 hours.
5. Aspirate medium from the shell vials and wash the coverslips 3X in PBS.
6. Add 1 mL of Carnoy’s fixative (3 parts methanol :1 part glacial acetic acid) to each shell vial, aspirate out and immediately add an additional 1 mL of Carnoy’s fixative. (The first prewash with Carnoy’s ensures good fixation as it removes any traces of water from the previous washing step.) Fix shell vials at –20°C for 20 minutes.
7. Aspirate fixative from the shell vials and wash coverslips 3X in PBS.
DNA Denaturation:
8. Cells are denatured by adding 0.2 mL of 2M HCL in water to each shell vial and incubating at 37°C for 1 hour.
9. Aspirate acid from the shell vials and neutralize the coverslips by washing 3X in borate buffer pH 8.5.
10. Wash shell vials 3X in PBS + 0.05% Tween 20 [PBS/T20], and stain or cap and store at 2–8°C.
Immunostaining:
11. Cells are blocked by adding 0.2 mL of PBS/T20/2% normal goat serum to each vial and incubating at 37°C for 10–30 minutes
12. Aspirate out shell vials. Add 0.2 mL of anti-BrdU monoclonal antibody [cat. No. MAB3424, MAB4072], diluted in PBS/T20/2% normal goat serum. Incubate vials at 37°C for 30 minutes
Note: BrdU stock solution: 10 mM dissolved in PBS, 0.2 µm filter sterilized and stored at –20°C. BrdU labeling solution: BrdU stock solution was diluted 1/100 in culture medium to yield a 100 µM solution (10X). The 10X solution was added to each shell vial to a final concentration of 10 µM.
13. Wash shell vials 3X in PBS/T20, and add 0.2 mL of goat anti mouse FITC [cat. No. AP124F] conjugate in PBS/T20 to each vial [1:500–1:1000]. Incubate vials at 37°C for 30 minutes, in the dark.
14. Wash shell vials 3X in PBS/T20. Remove coverslips from the vials, dip in DI water and mount on glass slides, using mounting media for fluorescence [cat. No. 5013]. Prepared slides can be stored in a humid chamber, refrigerated and in the dark at least 2 hours to overnight.
15. Evaluate slides using fluorescence microscopy.







