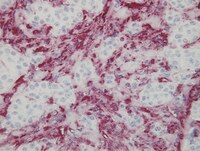
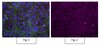
Platelets enhance tissue factor protein and metastasis initiating cell markers, and act as chemoattractants increasing the migration of ovarian cancer cells.
Orellana, R; Kato, S; Erices, R; Bravo, ML; Gonzalez, P; Oliva, B; Cubillos, S; Valdivia, A; Ibañez, C; Brañes, J; Barriga, MI; Bravo, E; Alonso, C; Bustamente, E; Castellon, E; Hidalgo, P; Trigo, C; Panes, O; Pereira, J; Mezzano, D; Cuello, MA; Owen, GI
BMC cancer
15
290
2015
Show Abstract
An increase in circulating platelets, or thrombocytosis, is recognized as an independent risk factor of bad prognosis and metastasis in patients with ovarian cancer; however the complex role of platelets in tumor progression has not been fully elucidated. Platelet activation has been associated with an epithelial to mesenchymal transition (EMT), while Tissue Factor (TF) protein expression by cancer cells has been shown to correlate with hypercoagulable state and metastasis. The aim of this work was to determine the effect of platelet-cancer cell interaction on TF and "Metastasis Initiating Cell (MIC)" marker levels and migration in ovarian cancer cell lines and cancer cells isolated from the ascetic fluid of ovarian cancer patients.With informed patient consent, ascitic fluid isolated ovarian cancer cells, cell lines and ovarian cancer spheres were co-cultivated with human platelets. TF, EMT and stem cell marker levels were determined by Western blotting, flow cytometry and RT-PCR. Cancer cell migration was determined by Boyden chambers and the scratch assay.The co-culture of patient-derived ovarian cancer cells with platelets causes: 1) a phenotypic change in cancer cells, 2) chemoattraction and cancer cell migration, 3) induced MIC markers (EMT/stemness), 3) increased sphere formation and 4) increased TF protein levels and activity.We present the first evidence that platelets act as chemoattractants to cancer cells. Furthermore, platelets promote the formation of ovarian cancer spheres that express MIC markers and the metastatic protein TF. Our results suggest that platelet-cancer cell interaction plays a role in the formation of metastatic foci. | | | 25886038
 |
An image-based, dual fluorescence reporter assay to evaluate the efficacy of shRNA for gene silencing at the single-cell level.
Kojima, S; Borisy, GG
F1000Research
3
60
2014
Show Abstract
RNA interference (RNAi) is widely used to suppress gene expression in a specific manner. The efficacy of RNAi is mainly dependent on the sequence of small interfering RNA (siRNA) in relation to the target mRNA. Although several algorithms have been developed for the design of siRNA, it is still difficult to choose a really effective siRNA from among multiple candidates. In this article, we report the development of an image-based, quantitative, ratiometric fluorescence reporter assay to evaluate the efficacy of RNAi at the single-cell level. Two fluorescence reporter constructs are used. One expresses the candidate small hairpin RNA (shRNA) together with an enhanced green fluorescent protein (EGFP); the other expresses a 19-nt target sequence inserted into a cassette expressing a red fluorescent protein (either DsRed or mCherry). Effectiveness of the candidate shRNA is evaluated as the extent to which it knocks down expression of the red fluorescent protein. Thus, the red-to-green fluorescence intensity ratio (appropriately normalized to controls) is used as the read-out for quantifying the siRNA efficacy at the individual cell level. We tested this dual fluorescence assay and compared predictions to actual endogenous knockdown levels for three different genes (vimentin, lamin A/C and Arp3) and twenty different shRNAs. For each of the genes, our assay successfully predicted the target sequences for effective RNAi. To further facilitate testing of RNAi efficacy, we developed a negative selection marker ( ccdB) method for construction of shRNA and red fluorescent reporter plasmids that allowed us to purify these plasmids directly from transformed bacteria without the need for colony selection and DNA sequencing verification. | Western Blotting | | 24741441
 |
Misfolded polyglutamine, polyalanine, and superoxide dismutase 1 aggregate via distinct pathways in the cell.
Polling, S; Mok, YF; Ramdzan, YM; Turner, BJ; Yerbury, JJ; Hill, AF; Hatters, DM
The Journal of biological chemistry
289
6669-80
2014
Show Abstract
Protein aggregation into intracellular inclusions is a key feature of many neurodegenerative disorders. A common theme has emerged that inappropriate self-aggregation of misfolded or mutant polypeptide sequences is detrimental to cell health. Yet protein quality control mechanisms may also deliberately cluster them together into distinct inclusion subtypes, including the insoluble protein deposit (IPOD) and the juxtanuclear quality control (JUNQ). Here we investigated how the intrinsic oligomeric state of three model systems of disease-relevant mutant protein and peptide sequences relates to the IPOD and JUNQ patterns of aggregation using sedimentation velocity analysis. Two of the models (polyalanine (37A) and superoxide dismutase 1 (SOD1) mutants A4V and G85R) accumulated into the same JUNQ-like inclusion whereas the other, polyglutamine (72Q), formed spatially distinct IPOD-like inclusions. Using flow cytometry pulse shape analysis (PulSA) to separate cells with inclusions from those without revealed the SOD1 mutants and 37A to have abruptly altered oligomeric states with respect to the nonaggregating forms, regardless of whether cells had inclusions or not, whereas 72Q was almost exclusively monomeric until inclusions formed. We propose that mutations leading to JUNQ inclusions induce a constitutively "misfolded" state exposing hydrophobic side chains that attract and ultimately overextend protein quality capacity, which leads to aggregation into JUNQ inclusions. Poly(Q) is not misfolded in this same sense due to universal polar side chains, but is highly prone to forming amyloid fibrils that we propose invoke a different engagement mechanism with quality control. | | | 24425868
 |
Distal renal tubules are deficient in aggresome formation and autophagy upon aldosterone administration.
Cheema, MU; Damkier, HH; Nielsen, J; Poulsen, ET; Enghild, JJ; Fenton, RA; Praetorius, J
PloS one
9
e101258
2014
Show Abstract
Prolonged elevations of plasma aldosterone levels are associated with renal pathogenesis. We hypothesized that renal distress could be imposed by an augmented aldosterone-induced protein turnover challenging cellular protein degradation systems of the renal tubular cells. Cellular accumulation of specific protein aggregates in rat kidneys was assessed after 7 days of aldosterone administration. Aldosterone induced intracellular accumulation of 60 s ribosomal protein L22 in protein aggregates, specifically in the distal convoluted tubules. The mineralocorticoid receptor inhibitor spironolactone abolished aldosterone-induced accumulation of these aggregates. The aldosterone-induced protein aggregates also contained proteasome 20 s subunits. The partial de-ubiquitinase ataxin-3 was not localized to the distal renal tubule protein aggregates, and the aggregates only modestly colocalized with aggresome transfer proteins dynactin p62 and histone deacetylase 6. Intracellular protein aggregation in distal renal tubules did not lead to development of classical juxta-nuclear aggresomes or to autophagosome formation. Finally, aldosterone treatment induced foci in renal cortex of epithelial vimentin expression and a loss of E-cadherin expression, as signs of cellular stress. The cellular changes occurred within high, but physiological aldosterone concentrations. We conclude that aldosterone induces protein accumulation in distal renal tubules; these aggregates are not cleared by autophagy that may lead to early renal tubular damage. | | | 25000288
 |
Neurosteroids are endogenous neuroprotectants in an ex vivo glaucoma model.
Ishikawa, M; Yoshitomi, T; Zorumski, CF; Izumi, Y
Investigative ophthalmology & visual science
55
8531-41
2014
Show Abstract
Allopregnanolone is a neurosteroid and powerful modulator of neuronal excitability. The neuroprotective effects of allopregnanolone involve potentiation of γ-aminobutyric acid (GABA) inhibitory responses. Although glutamate excitotoxicity contributes to ganglion cell death in glaucoma, the role of GABA in glaucoma remains uncertain. The aim of this study was to determine whether allopregnanolone synthesis is induced by high pressure in the retina and whether allopregnanolone modulates pressure-mediated toxicity.Ex vivo rat retinas were exposed to hydrostatic pressure (10, 35, and 75 mm Hg) for 24 hours. Endogenous allopregnanolone production was determined by liquid chromatography and tandem mass spectrometry (LC-MS/MS) and immunochemistry. We also examined the effects of allopregnanolone, finasteride, and dutasteride (inhibitors of 5α-reductase), picrotoxin (a GABA(A) receptor antagonist), and D-2-amino-5-phosphonovalerate (APV, a broad-spectrum N-methyl-D-aspartate receptor [NMDAR] antagonist).Pressure loading at 75 mm Hg significantly increased allopregnanolone levels as measured by LC-MS/MS. Elevated hydrostatic pressure also increased neurosteroid immunofluorescence, especially in the ganglion cell layer and inner nuclear layers. Staining was negligible at lower pressures. Enhanced allopregnanolone levels and immunostaining were substantially blocked by finasteride, but more effectively inhibited by dutasteride and APV. Administration of exogenous allopregnanolone suppressed pressure-induced axonal swelling in a concentration-dependent manner, while picrotoxin overcame these neuroprotective effects.These results indicate that the synthesis of allopregnanolone is enhanced mainly via NMDARs in the pressure-loaded retina, and that allopregnanolone diminishes pressure-mediated retinal degeneration via GABAA receptors. Allopregnanolone and other related neurosteroids may serve as potential novel therapeutic targets for the prevention of pressure-induced retinal damage in glaucoma. | | | 25406290
 |
Repair of astrocytes, blood vessels, and myelin in the injured brain: possible roles of blood monocytes.
Jeong, HK; Ji, KM; Kim, J; Jou, I; Joe, EH
Molecular brain
6
28
2013
Show Abstract
Inflammation in injured tissue has both repair functions and cytotoxic consequences. However, the issue of whether brain inflammation has a repair function has received little attention. Previously, we demonstrated monocyte infiltration and death of neurons and resident microglia in LPS-injected brains (Glia. 2007. 55:1577; Glia. 2008. 56:1039). Here, we found that astrocytes, oligodendrocytes, myelin, and endothelial cells disappeared in the damage core within 1-3 d and then re-appeared at 7-14 d, providing evidence of repair of the brain microenvironment. Since round Iba-1+/CD45+ monocytes infiltrated before the repair, we examined whether these cells were involved in the repair process. Analysis of mRNA expression profiles showed significant upregulation of repair/resolution-related genes, whereas proinflammatory-related genes were barely detectable at 3 d, a time when monocytes filled injury sites. Moreover, Iba-1+/CD45+ cells highly expressed phagocytic activity markers (e.g., the mannose receptors, CD68 and LAMP2), but not proinflammatory mediators (e.g., iNOS and IL1β). In addition, the distribution of round Iba-1+/CD45+ cells was spatially and temporally correlated with astrocyte recovery. We further found that monocytes in culture attracted astrocytes by releasing soluble factor(s). Together, these results suggest that brain inflammation mediated by monocytes functions to repair the microenvironment of the injured brain. | | | 23758980
 |
Improving outcomes of acute kidney injury using mouse renal progenitor cells alone or in combination with erythropoietin or suramin.
Han, X; Zhao, L; Lu, G; Ge, J; Zhao, Y; Zu, S; Yuan, M; Liu, Y; Kong, F; Xiao, Z; Zhao, S
Stem cell research & therapy
4
74
2013
Show Abstract
So far, no effective therapy is available for acute kidney injury (AKI), a common and serious complication with high morbidity and mortality. Interest has recently been focused on the potential therapeutic effect of mouse adult renal progenitor cells (MRPC), erythropoietin (EPO) and suramin in the recovery of ischemia-induced AKI. The aim of the present study is to compare MRPC with MRPC/EPO or MRPC/suramin concomitantly in the treatment of a mouse model of ischemia/reperfusion (I/R) AKI.MRPC were isolated from adult C57BL/6-gfp mice. Male C57BL/6 mice (eight-weeks old, n = 72) were used for the I/R AKI model. Serum creatinine (Cr), blood urea nitrogen (BUN) and renal histology were detected in MRPC-, MRPC/EPO-, MRPC/suramin- and PBS-treated I/R AKI mice. E-cadherin, CD34 and GFP protein expression was assessed by immunohistochemical assay.MRPC exhibited characteristics consistent with renal stem cells. The features of MRPC were manifested by Pax-2, Oct-4, vimentin, α-smooth muscle actin positive, and E-cadherin negative, distinguished from mesenchymal stem cells (MSC) by expression of CD34 and Sca-1. The plasticity of MRPC was shown by the ability to differentiate into osteoblasts and lipocytes in vitro. Injection of MRPC, especially MRPC/EPO and MRPC/suramin in I/R AKI mice attenuated renal damage with a decrease of the necrotic injury, peak plasma Cr and BUN. Furthermore, seven days after the injury, MRPC/EPO or MRPC/suramin formed more CD34(+) and E-cadherin+ cells than MRPC alone.These results suggest that MRPC, in particular MRPC/EPO or MRPC/suramin, promote renal repair after injury and may be a promising therapeutic strategy. | | | 23777889
 |
Aldosterone and angiotensin II induce protein aggregation in renal proximal tubules.
Cheema, MU; Poulsen, ET; Enghild, JJ; Hoorn, EJ; Hoorn, E; Fenton, RA; Praetorius, J
Physiological reports
1
e00064
2013
Show Abstract
Renal tubules are highly active transporting epithelia and are at risk of protein aggregation due to high protein turnover and/or oxidative stress. We hypothesized that the risk of aggregation was increased upon hormone stimulation and assessed the state of the intracellular protein degradation systems in the kidney from control rats and rats receiving aldosterone or angiotensin II treatment for 7 days. Control rats formed both aggresomes and autophagosomes specifically in the proximal tubules, indicating a need for these structures even under baseline conditions. Fluorescence sorted aggresomes contained various rat keratins known to be expressed in renal tubules as assessed by protein mass spectrometry. Aldosterone administration increased the abundance of the proximal tubular aggresomal protein keratin 5, the ribosomal protein RPL27, ataxin-3, and the chaperone heat shock protein 70-4 with no apparent change in the aggresome-autophagosome markers. Angiotensin II induced aggregation of RPL27 specifically in proximal tubules, again without apparent change in antiaggregating proteins or the aggresome-autophagosome markers. Albumin endocytosis was unaffected by the hormone administration. Taken together, we find that the renal proximal tubules display aggresome formation and autophagy. Despite an increase in aggregation-prone protein load in these tubules during hormone treatment, renal proximal tubules seem to have sufficient capacity for removing protein aggregates from the cells. | | | 24303148
 |
Spontaneous generation of germline characteristics in mouse fibrosarcoma cells.
Ma, Z; Hu, Y; Jiang, G; Hou, J; Liu, R; Lu, Y; Liu, C
Scientific reports
2
743
2011
Show Abstract
Germline/embryonic-specific genes have been found to be activated in somatic tumors. In this study, we further showed that cells functioning as germline could be present in mouse fibrosarcoma cells (L929 cell line). Early germline-like cells spontaneously appeared in L929 cells and further differentiated into oocyte-like cells. These germline-like cells can, in turn, develop into blastocyst-like structures in vitro and cause teratocarcinomas in vivo, which is consistent with natural germ cells in function. Generation of germline-like cells from somatic tumors might provide a novel way to understand why somatic cancer cells have strong features of embryonic/germline development. It is thought that the germline traits of tumors are associated with the central characteristics of malignancy, such as immortalization, invasion, migration and immune evasion. Therefore, germline-like cells in tumors might provide potential targets to tumor biology, diagnosis and therapy. | | | 23077727
 |
Survival of cancer stem cells under hypoxia and serum depletion via decrease in PP2A activity and activation of p38-MAPKAPK2-Hsp27.
Lin, SP; Lee, YT; Wang, JY; Miller, SA; Chiou, SH; Hung, MC; Hung, SC
PloS one
7
e49605
2011
Show Abstract
Hypoxia and serum depletion are common features of solid tumors that occur upon antiangiogenesis, irradiation and chemotherapy across a wide variety of malignancies. Here we show that tumor cells expressing CD133, a marker for colorectal cancer initiating or stem cells, are enriched and survive under hypoxia and serum depletion conditions, whereas CD133- cells undergo apoptosis. CD133+ tumor cells increase cancer stem cell and epithelial-mesenchymal transition properties. Moreover, via screening a panel of tyrosine and serine/threonine kinase pathways, we identified Hsp27 is constitutively activated in CD133+ cells rather than CD133- cell under hypoxia and serum depletion conditions. However, there was no difference in Hsp27 activation between CD133+ and CD133- cells under normal growth condition. Hsp27 activation, which was mediated by the p38MAPK-MAPKAPK2-Hsp27 pathway, is required for CD133+ cells to inhibit caspase 9 and 3 cleavage. In addition, inhibition of Hsp27 signaling sensitizes CD133+ cells to hypoxia and serum depletion -induced apoptosis. Moreover, the antiapoptotic pathway is also activated in spheroid culture-enriched CD133+ cancer stem cells from a variety of solid tumor cells including lung, brain and oral cancer, suggesting it is a common pathway activated in cancer stem cells from multiple tumor types. Thus, activation of PP2A or inactivation of the p38MAPK-MAPKAPK2-Hsp27 pathway may develop new strategies for cancer therapy by suppression of their TIC population. | Western Blotting | | 23185379
 |