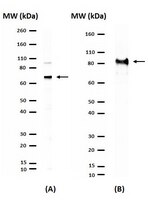
Regulation of somatostatin receptor 4-mediated cytostatic effects by CD26 in malignant pleural mesothelioma.
Yamamoto, J; Ohnuma, K; Hatano, R; Okamoto, T; Komiya, E; Yamazaki, H; Iwata, S; Dang, NH; Aoe, K; Kishimoto, T; Yamada, T; Morimoto, C
British journal of cancer
110
2232-45
2014
Show Abstract
Malignant pleural mesothelioma (MPM) is an aggressive neoplasm arising from mesothelial lining of pleura. CD26 molecules preferentially expressed on epithelioid type of MPM. This study investigates the molecular mechanisms of CD26 regulating MPM cells in vitro and in vivo.Biochemical and cell biological approaches were used for identifying a novel molecular target of MPM. Its contribution to tumour expansion has been also assessed using animal models. The clinical samples of MPM were also assessed for its expression.We identify that cytostatic effects in MPM are mediated by somatostatin (SST) receptor 4 (SSTR4), being inhibited by the interaction of CD26 molecules. We also indicates that SSTR4-mediated cytostatic effects are regulated by SHP-2 PTP, and that this inhibitory effect by SST agonist is enhanced via lipid raft clustering of associated molecules following crosslinking of anti-CD26 antibody. Finally, using an in vivo xenograft model, we demonstrate that the anti-tumour effect of anti-CD26 mAb is enhanced when combined with SSTR4 agonist treatment, and that SSTR4 is highly coexpressed with CD26 on epithelioid or biphasic types of MPM tissues obtained from patients' surgical specimens.Combination therapy with humanised anti-CD26 mAb and SSTR4 agonist may therefore potentiate anti-tumour effect on MPM. | | 24743707
 |
A hallmark of immunoreceptor, the tyrosine-based inhibitory motif ITIM, is present in the G protein-coupled receptor OX1R for orexins and drives apoptosis: a novel mechanism.
Thierry Voisin, Aadil El Firar, Christiane Rouyer-Fessard, Valérie Gratio, Marc Laburthe
The FASEB journal : official publication of the Federation of American Societies for Experimental Biology
22
1993-2002
2008
Show Abstract
Orexins acting at the G protein-coupled receptor (GPCR) OX1R have recently been shown to promote dramatic apoptosis in cancer cells. We report here that orexin-induced apoptosis is driven by an immunoreceptor tyrosine-based inhibitory motif (ITIM) (IIY(358)NFL) present in the OX1R. This effect is mediated by SHP-2 phosphatase recruitment via a mechanism that requires Gq protein but is independent of phospholipase C activation. This is based on the following observations: 1) mutation of Y(358) into F abolished orexin-induced tyrosine phosphorylation in ITIM, orexin-induced apoptosis, and uncoupled OX1R from Gq protein in transfected Chinese hamster ovary (CHO) cells; 2) orexin-induced apoptosis in CHO cells expressing recombinant OX1R and in colon cancer cells expressing the native receptor was abolished by treatment with the tyrosine phosphatase inhibitor PAO and by transfection with a dominant-negative mutant of SHP-2; 3) orexins were unable to promote apoptosis in fibroblast cells invalidated for the G alpha q subunit and transfected with OX1R cDNA, whereas they promoted apoptosis in cells equipped with G alpha q and OX1R; and 4) the phospholipase C inhibitor U-73122 blocked orexin-stimulated inositol phosphate formation, whereas it had no effect on orexin-induced apoptosis in CHO cells expressing OX1R. These data unravel a novel mechanism, whereby ITIM-expressing GPCRs may trigger apoptosis. | | 18198212
 |
An essential function for beta-arrestin 2 in the inhibitory signaling of natural killer cells.
Ming-Can Yu, Liu-Li Su, Lin Zou, Ye Liu, Na Wu, Ling Kong, Zi-Heng Zhuang, Lei Sun, Hai-Peng Liu, Jun-Hao Hu, Dangsheng Li, Jack L Strominger, Jing-Wu Zang, Gang Pei, Bao-Xue Ge
Nature immunology
9
898-907
2008
Show Abstract
The inhibitory signaling of natural killer (NK) cells is crucial in the regulation of innate immune responses. Here we show that the association of KIR2DL1, an inhibitory receptor of NK cells, with beta-arrestin 2 mediated recruitment of the tyrosine phosphatases SHP-1 and SHP-2 to KIR2DL1 and facilitated 'downstream' inhibitory signaling. Consequently, the cytotoxicity of NK cells was higher in beta-arrestin 2-deficient mice but was inhibited in beta-arrestin 2-transgenic mice. Moreover, beta-arrestin 2-deficient mice were less susceptible than wild-type mice to mouse cytomegalovirus infection, an effect that was abolished by depletion of NK cells. Our findings identify a previously unknown mechanism by which the inhibitory signaling in NK cells is regulated. | | 18604210
 |
Erythropoietin induces sustained phosphorylation of STAT5 in primitive but not definitive erythrocytes generated from mouse embryonic stem cells.
Kazue Tsuji-Takayama, Takeshi Otani, Toshiya Inoue, Shuji Nakamura, Ryuichi Motoda, Masayoshi Kibata, Kunzo Orita
Experimental hematology
34
1323-32
2005
Show Abstract
OBJECTIVE: During embryonic development murine erythropoiesis occurs in two waves by producing first primitive erythroid cells (EryPs) and then definitive erythroid cells (EryDs). Erythropoietin (EPO) signaling is compared between EryPs and EryDs. METHODS: We studied the EPO signaling in EryPs and EryDs using an embryonic stem-derived culture system, which can recapitulate this in vivo development process and has thus been used as a convenient in vitro model system of erythropoiesis. RESULTS: We found that EPO induced sustained phosphorylation and nuclear translocation of signal transducer and activator of transcription 5 (STAT5) in EryPs but not EryDs. EryPs expressed dramatically higher amounts of EPO receptor compared with EryDs, indicating there was excessive signaling from the receptor upon EPO stimulation. In addition, reduced expression of tyrosine phosphatase, Src homology region 2 domain-containing phosphatase-1, and decreased total phosphatase activity in EryPs partly explain the persistent activation of STAT5. Nevertheless, Janus kinase 2 (JAK2) phosphorylation, which is essential for transduction of EPO signaling from the EPO receptor to STAT5, was observed in a transient but not a persistent manner. Inhibition of JAK activity resulted in partial suppression of transient phosphorylation of STAT5 and no suppression of sustained phosphorylation of STAT5. CONCLUSION: This study presents a unique feature of EryPs, as this is the first known example of sustained activation of STAT5 in normal cells. Our results also imply the existence of a JAK2-independent pathway of EPO signaling to induce STAT5 activation. | | 16982325
 |
Molecular basis of the recruitment of the SH2 domain-containing inositol 5-phosphatases SHIP1 and SHIP2 by fcgamma RIIB.
Bruhns, P, et al.
J. Biol. Chem., 275: 37357-64 (2000)
1999
Show Abstract
FcgammaRIIB are single-chain low affinity receptors for IgG that negatively regulate immunoreceptor tyrosine-based activation motif-dependent cell activation. They bear one immunoreceptor tyrosine-based inhibition motif (ITIM) that becomes tyrosyl-phosphorylated upon coaggregation of FcgammaRIIB with immunoreceptor tyrosine-based activation motif-bearing receptors and that recruits SH2 domain-containing inositol 5-phosphatases (SHIPs) in vivo. Synthetic FcgammaRIIB ITIM phosphopeptides, however, also bind SH2 domain-containing protein-tyrosine phosphatases (SHPs) in vitro. To identify SHIP-binding sites, we exchanged residues between the FcgammaRIIB ITIM and the N-terminal ITIM of a killer cell Ig-like receptor that does not bind SHIPs. Loss of function and gain of function substitutions identified the Y+2 leucine, in the FcgammaRIIB ITIM, as determining the binding of both SHIP1 and SHIP2, but not the binding of SHP-1 or SHP-2. Conversely, the Y-2 isoleucine that determines the in vitro binding of SHP-1 and SHP-2 affected neither the binding nor the recruitment of SHIP1 or SHIP2. One hydrophobic residue, in the ITIM of FcgammaRIIB therefore determines the affinity for SHIPs. This residue is symmetrical to the hydrophobic residue that determines the affinity of all ITIMs for SHPs. It defines a SHIP-binding site, distinct from a SHP-binding site, that enables FcgammaRIIB to recruit SHIP1 and SHIP2 and that is preferentially used in vivo. | | 11016922
 |
The phosphotyrosine phosphatase PTP1D, but not PTP1C, is an essential mediator of fibroblast proliferation induced by tyrosine kinase and G protein-coupled receptors.
Rivard, N, et al.
J. Biol. Chem., 270: 11017-24 (1995)
1994
Show Abstract
PTP1C and PTP1D are non-transmembrane protein-tyrosine phosphatases (PTPs), which contain two src homology-2 domains. These enzymes are believed to play a role in regulating downstream signaling from receptors with intrinsic tyrosine kinase activity. The present study describes the tyrosine phosphorylation and the catalytic activity of both PTPs in CCL39 cells, a Chinese hamster lung fibroblast cell line, upon addition of a variety of growth factors. We demonstrate that PTP1C activity was significantly stimulated by insulin and the phorbol ester 12-O-tetradecanoylphorbol-13-acetate but was not influenced by serum, platelet-derived growth factor (PDGF), or alpha-thrombin. However, tyrosine phosphorylation of PTP1C was increased in response to insulin, PDGF, and alpha-thrombin. PTP1D activity was slightly stimulated by insulin and 12-O-tetradecanoylphorbol-13-acetate but was significantly inhibited by serum, PDGF, and alpha-thrombin, although tyrosine phosphorylation is increased in response to these agonists. Mitogen-activated protein kinase phosphorylated PTP1C and PTP1D in in vitro kinase assays, suggesting that both PTPs are target proteins for mitogen-activated protein kinase. We also show that overexpression of PTP1C or PTP1D had no effect on DNA synthesis stimulated by different growth factors. However, a mutated inactive form of PTP1D strongly inhibited the stimulatory effects of both PDGF and alpha-thrombin on early gene transcription and DNA synthesis. These results demonstrate for the first time that PTP1C and PTP1D may participate in signal transduction but in different manners and that only PTP1D is a positive mediator of mitogenic signals induced by both tyrosine kinase receptors and G protein-coupled receptors in fibroblasts. | Immunoprecipitation, Phosphatase Assay | 7537742
 |