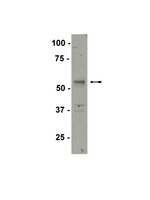
p54nrb/NONO regulates cyclic AMP-dependent glucocorticoid production by modulating phosphodiesterase mRNA splicing and degradation.
Lu, JY; Sewer, MB
Mol Cell Biol
35
1223-37
2015
Show Abstract
Glucocorticoid production in the adrenal cortex is activated in response to an increase in cyclic AMP (cAMP) signaling. The nuclear protein p54(nrb)/NONO belongs to the Drosophila behavior/human splicing (DBHS) family and has been implicated in several nuclear processes, including transcription, splicing, and RNA export. We previously identified p54(nrb)/NONO as a component of a protein complex that regulates the transcription of CYP17A1, a gene required for glucocorticoid production. Based on the multiple mechanisms by which p54(nrb)/NONO has been shown to control gene expression and the ability of the protein to be recruited to the CYP17A1 promoter, we sought to further define the molecular mechanism by which p54(nrb)/NONO confers optimal cortisol production. We show here that silencing p54(nrb)/NONO expression in H295R human adrenocortical cells decreases the ability of the cells to increase intracellular cAMP production and subsequent cortisol biosynthesis in response to adrenocorticotropin hormone (ACTH) stimulation. Interestingly, the expression of multiple phosphodiesterase (PDE) isoforms, including PDE2A, PDE3A, PDE3B, PDE4A, PDE4D, and PDE11A, was induced in p54(nrb)/NONO knockdown cells. Investigation of the mechanism by which silencing of p54(nrb)/NONO led to increased expression of select PDE isoforms revealed that p54(nrb)/NONO regulates the splicing of a subset of PDE isoforms. Importantly, we also identify a role for p54(nrb)/NONO in regulating the stability of PDE transcripts by facilitating the interaction between the exoribonuclease XRN2 and select PDE transcripts. In summary, we report that p54(nrb)/NONO modulates cAMP-dependent signaling, and ultimately cAMP-stimulated glucocorticoid biosynthesis by regulating the splicing and degradation of PDE transcripts. | | | 25605330
 |
Silencing diacylglycerol kinase-theta expression reduces steroid hormone biosynthesis and cholesterol metabolism in human adrenocortical cells.
Cai, K; Lucki, NC; Sewer, MB
Biochim Biophys Acta
1841
552-62
2014
Show Abstract
Diacylglycerol kinase theta (DGKθ) plays a pivotal role in regulating adrenocortical steroidogenesis by synthesizing the ligand for the nuclear receptor steroidogenic factor 1 (SF1). In response to activation of the cAMP signaling cascade nuclear DGK activity is rapidly increased, facilitating PA-mediated, SF1-dependent transcription of genes required for cortisol and dehydroepiandrosterone (DHEA) biosynthesis. Based on our previous work identifying DGKθ as the enzyme that produces the agonist for SF1, we generated a tetracycline-inducible H295R stable cell line to express a short hairpin RNA (shRNA) against DGKθ and characterized the effect of silencing DGKθ on adrenocortical gene expression. Genome-wide DNA microarray analysis revealed that silencing DGKθ expression alters the expression of multiple genes, including steroidogenic genes, nuclear receptors and genes involved in sphingolipid, phospholipid and cholesterol metabolism. Interestingly, the expression of sterol regulatory element binding proteins (SREBPs) was also suppressed. Consistent with the suppression of SREBPs, we observed a down-regulation of multiple SREBP target genes, including 3-hydroxy-3-methylglutary coenzyme A reductase (HMG-CoA red) and CYP51, concomitant with a decrease in cellular cholesterol. DGKθ knockdown cells exhibited a reduced capacity to metabolize PA, with a down-regulation of lipin and phospholipase D (PLD) isoforms. In contrast, suppression of DGKθ increased the expression of several genes in the sphingolipid metabolic pathway, including acid ceramidase (ASAH1) and sphingosine kinases (SPHK). In summary, these data demonstrate that DGKθ plays an important role in steroid hormone production in human adrenocortical cells. | Western Blotting | | 24369117
 |
Hormonal regulation of microRNA expression in steroid producing cells of the ovary, testis and adrenal gland.
Hu, Z; Shen, WJ; Cortez, Y; Tang, X; Liu, LF; Kraemer, FB; Azhar, S
PloS one
8
e78040
2013
Show Abstract
Given the emerging roles of miRNAs as potential posttranscriptional/posttranslational regulators of the steroidogenic process in adrenocortical and gonadal cells, we sought to determine miRNA profiles in rat adrenals from animals treated with vehicle, ACTH, 17α-E2 or dexamethasone. Key observations were also confirmed using hormone (Bt2cAMP)-treated mouse Leydig tumor cells, MLTC-1, and primary rat ovarian granulosa cells.RNA was extracted from rat adrenal glands and miRNA profiles were established using microarray and confirmed with qRT-PCR. The expression of some of the hormone-sensitive miRNAs was quantified in MLTC-1 and granulosa cells after stimulation with Bt2cAMP. Targets of hormonally altered miRNAs were explored by qRT-PCR and Western blotting in adrenals and granulosa cells.Adrenals from ACTH, 17α-E2 and dexamethasone treated rats exhibited miRNA profiles distinct from control animals. ACTH up-regulated the expression of miRNA-212, miRNA-182, miRNA-183, miRNA-132, and miRNA-96 and down-regulated the levels of miRNA-466b, miRNA-214, miRNA-503, and miRNA-27a. The levels of miR-212, miRNA-183, miRNA-182, miRNA-132, miRNA-370, miRNA-377, and miRNA-96 were up-regulated, whereas miR-125b, miRNA-200b, miR-122, miRNA-466b, miR-138, miRNA-214, miRNA-503 and miRNA27a were down-regulated in response to 17α-E2 treatment. Dexamethasone treatment decreased miRNA-200b, miR-122, miR-19a, miRNA-466b and miRNA27a levels, but increased miRNA-183 levels. Several adrenal miRNAs are subject to regulation by more than one hormone. Significant cAMP-induced changes in certain miRNAs were also noted in MLTC-1 and granulosa cells. Some of the hormone-induced miRNAs in steroidogenic cells were predicted to target proteins involved in lipid metabolism/steroidogenesis. We also obtained evidence that miR-132 and miRNA-214 inhibit the expression of SREBP-1c and LDLR, respectively.Our results demonstrate that expression of a number of miRNAs in steroidogenic cells of the testis, ovary and adrenal glands is subject to hormonal regulation and that miRNAs and their regulation by specific hormones are likely to play a key role in posttranscriptional/posttranslational regulation of steroidogenesis. | Western Blotting | | 24205079
 |
Integrative analysis of SF-1 transcription factor dosage impact on genome-wide binding and gene expression regulation.
Doghman, M; Figueiredo, BC; Volante, M; Papotti, M; Lalli, E
Nucleic acids research
41
8896-907
2013
Show Abstract
Steroidogenic Factor-1 (SF-1) is a nuclear receptor that has a pivotal role in the development of adrenal glands and gonads and in the control of steroid hormone production, being also implicated in the pathogenesis of adrenocortical tumors. We have analyzed the mechanisms how SF-1 controls gene expression in adrenocortical cells and showed that it regulates different categories of genes according to its dosage. Significant correlations exist between the localization of SF-1-binding sites in chromatin under different dosage conditions and dosage-dependent regulation of gene expression. Our study revealed unexpected functional interactions between SF-1 and Neuron-Restrictive Silencer Factor/RE1-Silencing Transcription Factor (NRSF/REST), which was first characterized as a repressor of neuronal gene expression in non-neuronal tissues, in the regulation of gene expression in steroidogenic cells. When overexpressed, SF-1 reshapes the repertoire of NRSF/REST-regulated genes, relieving repression of key steroidogenic genes. These data show that NRSF/REST has a novel function in regulating gene expression in steroidogenic cells and suggest that it may have a broad role in regulating tissue-specific gene expression programs. | | | 23907384
 |
Identification of transcription factors and coactivators affected by dibutylphthalate interactions in fetal rat testes.
Plummer, SM; Dan, D; Quinney, J; Hallmark, N; Phillips, RD; Millar, M; Macpherson, S; Elcombe, CR
Toxicological sciences : an official journal of the Society of Toxicology
132
443-57
2013
Show Abstract
Previous analysis of in utero dibutylphthalate (DBP)-exposed fetal rat testes indicated that DBP's antiandrogenic effects were mediated, in part, by indirect inhibition of steroidogenic factor 1 (SF1), suggesting that peroxisome proliferator-activated receptor alpha (PPARα) might be involved through coactivator (CREB-binding protein [CBP]) sequestration. To test this hypothesis, we have performed chromatin immunoprecipitation (ChIP) microarray analysis to assess the DNA binding of PPARα, SF1, CBP, and RNA polymerase II in DBP-induced testicular maldevelopment target genes. Pathway analysis of expression array data in fetal rat testes examined at gestational day (GD) 15, 17, or 19 indicated that lipid metabolism genes regulated by SF1 and PPARα, respectively, were overrepresented, and the time dependency of changes to PPARα-regulated lipid metabolism genes correlated with DBP-mediated repression of SF1-regulated steroidogenesis genes. ChIP microarrays were used to investigate whether DBP-mediated repression of SF1-regulated genes was associated with changes in SF1 binding to genes involved in DBP-induced testicular maldevelopment. DBP treatment caused reductions in SF1 binding in CYP11a, StAR, and CYP17a. Follicle-stimulating hormone receptor (FSHR), regulated by SF1 but unaffected by DBP-treatment, also contained SF1-binding peaks, but DBP did not change this compared with control. GD15 and GD19 fetal testes contained PPARα protein-binding peaks in CYP11a, StAR, and CYP17a regulatory regions. In contrast to its repressive effect on SF1, DBP treatment caused increases in these peaks compared with control. PPARα-binding peaks in the FSHR promoter were not detected in GD15 samples. Hence, the repressive effect of DBP on SF1-regulated steroidogenic genes correlates with inhibition of SF1-DNA binding and increased PPARα-DNA binding. The data indicate that PPARα may act as an indirect transrepressor of SF1 on steroidogenic genes in fetal rat testes in response to DBP treatment. | | | 23358192
 |
Acid ceramidase (ASAH1) is a global regulator of steroidogenic capacity and adrenocortical gene expression.
Lucki, NC; Bandyopadhyay, S; Wang, E; Merrill, AH; Sewer, MB
Mol Endocrinol
26
228-43
2011
Show Abstract
In H295R human adrenocortical cells, ACTH rapidly activates ceramide (Cer) and sphingosine (SPH) turnover with a concomitant increase in SPH-1-phosphate secretion. These bioactive lipids modulate adrenocortical steroidogenesis, primarily by acting as second messengers in the protein kinase A/cAMP-dependent pathway. Acid ceramidase (ASAH1) directly regulates the intracellular balance of Cer, SPH, and SPH-1-phosphate by catalyzing the hydrolysis of Cer into SPH. ACTH/cAMP signaling stimulates ASAH1 transcription and activity, supporting a role for this enzyme in glucocorticoid production. Here, the role of ASAH1 in regulating steroidogenic capacity was examined using a tetracycline-inducible ASAH1 short hairpin RNA H295R human adrenocortical stable cell line. We show that ASAH1 suppression increases the transcription of multiple steroidogenic genes, including Cytochrome P450 monooxygenase (CYP)17A1, CYP11B1/2, CYP21A2, steroidogenic acute regulatory protein, hormone-sensitive lipase, 18-kDa translocator protein, and the melanocortin-2 receptor. Induced gene expression positively correlated with enhanced histone H3 acetylation at target promoters. Repression of ASAH1 expression also induced the expression of members of the nuclear receptor nuclear receptor subfamily 4 (NR4A) family while concomitantly suppressing the expression of dosage-sensitive sex reversal, adrenal hypoplasia critical region, on chromosome X, gene 1. ASAH1 knockdown altered the expression of genes involved in sphingolipid metabolism and changed the cellular amounts of distinct sphingolipid species. Finally, ASAH1 silencing increased basal and cAMP-dependent cortisol and dehydroepiandrosterone secretion, establishing ASAH1 as a pivotal regulator of steroidogenic capacity in the human adrenal cortex. | | | 22261821
 |
Acid ceramidase (ASAH1) represses steroidogenic factor 1-dependent gene transcription in H295R human adrenocortical cells by binding to the receptor.
Lucki, NC; Li, D; Bandyopadhyay, S; Wang, E; Merrill, AH; Sewer, MB
Mol Cell Biol
32
4419-31
2011
Show Abstract
Adrenocorticotropin (ACTH) signaling increases glucocorticoid production by promoting the interaction of transcription factors and coactivator proteins with the promoter of steroidogenic genes. The nuclear receptor steroidogenic factor 1 (SF-1) is essential for steroidogenic gene transcription. Sphingosine (SPH) is a ligand for SF-1. Moreover, suppression of expression of acid ceramidase (ASAH1), an enzyme that produces SPH, increases the transcription of multiple steroidogenic genes. Given that SF-1 is a nuclear protein, we sought to define the molecular mechanisms by which ASAH1 regulates SF-1 function. We show that ASAH1 is localized in the nuclei of H295R adrenocortical cells and that cyclic AMP (cAMP) signaling promotes nuclear sphingolipid metabolism in an ASAH1-dependent manner. ASAH1 suppresses SF-1 activity by directly interacting with the receptor. Chromatin immunoprecipitation (ChIP) assays revealed that ASAH1 is recruited to the promoter of various SF-1 target genes and that ASAH1 and SF-1 colocalize on the same promoter region of the CYP17A1 and steroidogenic acute regulatory protein (StAR) genes. Taken together, these results demonstrate that ASAH1 is a novel coregulatory protein that represses SF-1 function by directly binding to the receptor on SF-1 target gene promoters and identify a key role for nuclear lipid metabolism in regulating gene transcription. | | | 22927646
 |
Nuclear receptor 5A (NR5A) family regulates 5-aminolevulinic acid synthase 1 (ALAS1) gene expression in steroidogenic cells.
Ju, Y; Mizutani, T; Imamichi, Y; Yazawa, T; Matsumura, T; Kawabe, S; Kanno, M; Umezawa, A; Kangawa, K; Miyamoto, K
Endocrinology
153
5522-34
2011
Show Abstract
5-Aminolevulinic acid synthase 1 (ALAS1) is a rate-limiting enzyme for heme biosynthesis in mammals. Heme is essential for the catalytic activities of P450 enzymes including steroid metabolic enzymes. Nuclear receptor 5A (NR5A) family proteins, steroidogenic factor-1 (SF-1), and liver receptor homolog-1 (LRH-1) play pivotal roles in regulation of steroidogenic enzymes. Recently, we showed that expression of SF-1/LRH-1 induces differentiation of mesenchymal stem cells into steroidogenic cells. In this study, genome-wide analysis revealed that ALAS1 was a novel SF-1-target gene in differentiated mesenchymal stem cells. Chromatin immunoprecipitation and reporter assays revealed that SF-1/LRH-1 up-regulated ALAS1 gene transcription in steroidogenic cells via binding to a 3.5-kb upstream region of ALAS1. The ALAS1 gene was up-regulated by overexpression of SF-1/LRH-1 in steroidogenic cells and down-regulated by knockdown of SF-1 in these cells. Peroxisome proliferator-activated receptor-γ coactivator-1α, a coactivator of nuclear receptors, also strongly coactivated expression of NR5A-target genes. Reporter analysis revealed that peroxisome proliferator-activated receptor-γ coactivator-1α strongly augmented ALAS1 gene transcription caused by SF-1 binding to the 3.5-kb upstream region. Finally knockdown of ALAS1 resulted in reduced progesterone production by steroidogenic cells. These results indicate that ALAS1 is a novel NR5A-target gene and participates in steroid hormone production. | | | 23024262
 |
KIAA0101 is overexpressed, and promotes growth and invasion in adrenal cancer.
Jain, M; Zhang, L; Patterson, EE; Kebebew, E
PloS one
6
e26866
2010
Show Abstract
KIAA0101 is a proliferating cell nuclear antigen-associated factor that is overexpressed in some human malignancies. Adrenocortical neoplasm is one of the most common human neoplasms for which the molecular causes are poorly understood. Moreover, it is difficult to distinguish between localized benign and malignant adrenocortical tumors. For these reasons, we studied the expression, function and possible mechanism of dysregulation of KIAA0101 in human adrenocortical neoplasm.KIAA0101 mRNA and protein expression levels were determined in 112 adrenocortical tissue samples (21 normal adrenal cortex, 80 benign adrenocortical tumors, and 11 adrenocortical carcinoma (ACC). SiRNA knockdown was used to determine the functional role of KIAA0101 on cell proliferation, cell cycle, apoptosis, soft agar anchorage independent growth and invasion in the ACC cell line, NCI-H295R. In addition, we explored the mechanism of KIAA0101 dysregulation by examining the mutational status. KIAA0101 mRNA (9.7 fold) and protein expression were significantly higher in ACC (pless than 0.0001). KIAA0101 had sparse protein expression in only a few normal adrenal cortex samples, which was confined to adrenocortical progenitor cells. KIAA0101 expression levels were 84% accurate for distinguishing between ACC and normal and benign adrenocortical tumor samples. Knockdown of KIAA0101 gene expression significantly decreased anchorage independent growth by 80% and invasion by 60% (p = 0.001; p = 0.006). We found no mutations in KIAA0101 in ACC.KIAA0101 is overexpressed in ACC. Our data supports that KIAA0101 is a marker of cellular proliferation, promotes growth and invasion, and is a good diagnostic marker for distinguishing benign from malignant adrenocortical neoplasm. | Immunofluorescence | Human | 22096502
 |
BRCA1/BARD1 complex interacts with steroidogenic factor 1--A potential mechanism for regulation of aromatase expression by BRCA1.
Lu, Y; Kang, T; Hu, Y
The Journal of steroid biochemistry and molecular biology
123
71-8
2010
Show Abstract
Germline mutations in BRCA1 predispose women to early onset of breast and ovarian cancers. Findings from previous studies support the notion that the tissue- and gender-specific tumor suppression function of BRCA1 is associated with its role in negative regulation of aromatase expression, the rate-limiting step in estrogen biosynthesis. The molecular mechanism of BRCA1 in regulating aromatase promoter activity remains to be elucidated. In this study, we demonstrate that, in an ovarian granulosa cell line KGN, steroidogenic factor 1 (SF-1) is required for aromatase PII promoter basal activity as well as the elevated aromatase expression mediated by BRCA1 knockdown. Furthermore, BRCA1 in KGN cells exists mainly as a heterodimer with BARD1. We provide evidence that the BRCA1/BARD1 complex interacts with SF-1 both in vivo and in vitro. However, the intrinsic ubiquitin E3 ligase activity of BRCA1/BARD1 does not appear to contribute to ubiquitynation of SF-1. We propose that the interaction between SF-1 and BRCA1/BARD1 may recruit BRCA1/BARD1 complex to the aromatase PII promoter for BRCA1/BARD1-mediate transcriptional repression. Full Text Article | | | 21087664
 |