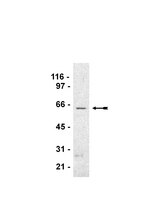
Cdk1 phosphorylates the Rac activator Tiam1 to activate centrosomal Pak and promote mitotic spindle formation.
Whalley, HJ; Porter, AP; Diamantopoulou, Z; White, GR; Castañeda-Saucedo, E; Malliri, A
Nature communications
6
7437
2015
Show Abstract
Centrosome separation is critical for bipolar spindle formation and the accurate segregation of chromosomes during mammalian cell mitosis. Kinesin-5 (Eg5) is a microtubule motor essential for centrosome separation, and Tiam1 and its substrate Rac antagonize Eg5-dependent centrosome separation in early mitosis promoting efficient chromosome congression. Here we identify S1466 of Tiam1 as a novel Cdk1 site whose phosphorylation is required for the mitotic function of Tiam1. We find that this phosphorylation of Tiam1 is required for the activation of group I p21-activated kinases (Paks) on centrosomes in prophase. Further, we show that both Pak1 and Pak2 counteract centrosome separation in a kinase-dependent manner and demonstrate that they act downstream of Tiam1. We also show that depletion of Pak1/2 allows cells to escape monopolar arrest by Eg5 inhibition, highlighting the potential importance of this signalling pathway for the development of Eg5 inhibitors as cancer therapeutics. | | 26078008
 |
Cell cycle-dependent localization of CHK2 at centrosomes during mitosis.
Chouinard, G; Clément, I; Lafontaine, J; Rodier, F; Schmitt, E
Cell division
8
7
2013
Show Abstract
Centrosomes function primarily as microtubule-organizing centres and play a crucial role during mitosis by organizing the bipolar spindle. In addition to this function, centrosomes act as reaction centers where numerous key regulators meet to control cell cycle progression. One of these factors involved in genome stability, the checkpoint kinase CHK2, was shown to localize at centrosomes throughout the cell cycle.Here, we show that CHK2 only localizes to centrosomes during mitosis. Using wild-type and CHK2-/- HCT116 human colon cancer cells and human osteosarcoma U2OS cells depleted for CHK2 with small hairpin RNAs we show that several CHK2 antibodies are non-specific and cross-react with an unknown centrosomal protein(s) by immunofluorescence. To characterize the localization of CHK2, we generated cells expressing inducible GFP-CHK2 and Flag-CHK2 fusion proteins. We show that CHK2 localizes to the nucleus in interphase cells but that a fraction of CHK2 associates with the centrosomes in a Polo-like kinase 1-dependent manner during mitosis, from early mitotic stages until cytokinesis.Our findings demonstrate that a subpopulation of CHK2 localizes at the centrosomes in mitotic cells but not in interphase. These results are consistent with previous reports supporting a role for CHK2 in the bipolar spindle formation and the timely progression of mitosis. | | 23680298
 |
Plk4 is required for cytokinesis and maintenance of chromosomal stability.
Carla O Rosario,Michael A Ko,Yosr Z Haffani,Rebecca A Gladdy,Jana Paderova,Aaron Pollett,Jeremy A Squire,James W Dennis,Carol J Swallow
Proceedings of the National Academy of Sciences of the United States of America
107
2009
Show Abstract
Aneuploidy is a characteristic feature of established cancers and can promote tumor development. Aneuploidy may arise directly, through unequal distribution of chromosomes into daughter cells, or indirectly, through a tetraploid intermediate. The polo family kinase Plk4/Sak is required for late mitotic progression and is haploinsufficient for tumor suppression in mice. Here we show that loss of heterozygosity (LOH) occurs at the Plk4 locus in 50% of human hepatocellular carcinomas (HCC) and is present even in preneoplastic cirrhotic liver nodules. LOH at Plk4 is associated with reduced Plk4 expression in HCC tumors but not with mutations in the remaining allele. Plk4(+/-) murine embryonic fibroblasts (MEFs) at early passage show a high incidence of multinucleation, supernumerary centrosomes, and a near-tetraploid karyotype. Underlying these phenotypes is a high rate of primary cytokinesis failure, associated with aberrant actomyosin ring formation, reduced RhoA activation, and failure to localize the RhoA guanine nucleotide exchange factor Ect2 to the spindle midbody. We further show that Plk4 normally localizes to the midbody and binds to and phosphorylates Ect2 in vitro. With serial passaging Plk4(+/-) MEFs rapidly immortalize, acquiring an increasing burden of nonclonal and clonal gross chromosomal irregularities, and form tumors in vivo. Our results indicate that haploid levels of Plk4 disrupt RhoGTPase function during cytokinesis, resulting in aneuploidy and tumorigenesis, thus implicating early LOH at Plk4 as one of the drivers of human hepatocellular carcinogenesis. These findings represent an advance in our understanding of genetic predisposition to HCC, which continues to increase in incidence globally and particularly in North America. Full Text Article | | 20348415
 |
Plk1 self-organization and priming phosphorylation of HsCYK-4 at the spindle midzone regulate the onset of division in human cells.
Burkard, ME; Maciejowski, J; Rodriguez-Bravo, V; Repka, M; Lowery, DM; Clauser, KR; Zhang, C; Shokat, KM; Carr, SA; Yaffe, MB; Jallepalli, PV
PLoS biology
7
e1000111
2009
Show Abstract
Animal cells initiate cytokinesis in parallel with anaphase onset, when an actomyosin ring assembles and constricts through localized activation of the small GTPase RhoA, giving rise to a cleavage furrow. Furrow formation relies on positional cues provided by anaphase spindle microtubules (MTs), but how such cues are generated remains unclear. Using chemical genetics to achieve both temporal and spatial control, we show that the self-organized delivery of Polo-like kinase 1 (Plk1) to the midzone and its local phosphorylation of a MT-bound substrate are critical for generating this furrow-inducing signal. When Plk1 was active but unable to target itself to this equatorial landmark, both cortical RhoA recruitment and furrow induction failed to occur, thus recapitulating the effects of anaphase-specific Plk1 inhibition. Using tandem mass spectrometry and phosphospecific antibodies, we found that Plk1 binds and directly phosphorylates the HsCYK-4 subunit of centralspindlin (also known as MgcRacGAP) at the midzone. At serine 157, this modification creates a major docking site for the tandem BRCT repeats of the Rho GTP exchange factor Ect2. Cells expressing only a nonphosphorylatable form of HsCYK-4 failed to localize Ect2 at the midzone and were severely impaired in cleavage furrow formation, implying that HsCYK-4 is Plk1's rate-limiting target upstream of RhoA. Conversely, tethering an inhibitor-resistant allele of Plk1 to HsCYK-4 allowed furrows to form despite global inhibition of all other Plk1 molecules in the cell. Our findings illuminate two key mechanisms governing the initiation of cytokinesis in human cells and illustrate the power of chemical genetics to probe such regulation both in time and space. | Western Blotting | 19468302
 |
Cell polarization during monopolar cytokinesis.
Hu, CK; Coughlin, M; Field, CM; Mitchison, TJ
The Journal of cell biology
181
195-202
2008
Show Abstract
During cytokinesis, a specialized set of proteins is recruited to the equatorial region between spindle poles by microtubules and actin filaments, enabling furrow assembly and ingression before cell division. We investigate the mechanisms underlying regional specialization of the cytoskeleton in HeLa cells undergoing drug-synchronized monopolar cytokinesis. After forced mitotic exit, the cytoskeleton of monopolar mitotic cells is initially radially symmetric but undergoes a symmetry-breaking reaction that simultaneously polarizes microtubules and the cell cortex, with a concentration of cortical furrow markers into a cap at one side of the cell. Polarization requires microtubules, F-actin, RhoA, Myosin II activity, and Aurora B kinase activity. Aurora B localizes to actin cables in a gap between the monopolar midzone and the furrow-like cortex, suggesting a communication between them. We propose that feedback loops between cortical furrow components and microtubules promote symmetry breaking during monopolar cytokinesis and regional specialization of the cytoskeleton during normal bipolar cytokinesis. Full Text Article | | 18411311
 |
The ubiquitin-conjugating enzyme E2-EPF is overexpressed in primary breast cancer and modulates sensitivity to topoisomerase II inhibition.
Tedesco, D; Zhang, J; Trinh, L; Lalehzadeh, G; Meisner, R; Yamaguchi, KD; Ruderman, DL; Dinter, H; Zajchowski, DA
Neoplasia (New York, N.Y.)
9
601-13
2007
Show Abstract
We identified the ubiquitin-conjugating enzyme E2-EPF mRNA as differentially expressed in breast tumors relative to normal tissues and performed studies to elucidate its putative role in cancer. We demonstrated that overexpression of E2-EPF protein correlated with estrogen receptor (ER) negativity in breast cancer specimens and that its expression is cell cycle-regulated, suggesting a potential function for E2-EPF in cell cycle progression. However, reduction of E2-EPF protein levels by greater than 80% using RNAi had no significant effects on the proliferation of HeLa cervical cancer cells or ER(-) MDA-MB-231 or MDA-MB-453 breast cancer cells. Because E2-EPF protein levels were elevated during the G(2)/M phase of the cell cycle and because E2-EPF mRNA in tumor specimens was frequently coexpressed with genes involved in cell cycle control, spindle assembly, and mitotic surveillance, the possibility that E2-EPF might have a function in the cellular response to agents that induce a G(2) checkpoint or an M checkpoint was investigated. E2-EPF knockdown sensitized HeLa cells to the topoisomerase (topo) II inhibitors etoposide and doxorubicin and also increased topo IIalpha protein levels. These data suggest that combined administration of topo II-directed drugs and E2-EPF inhibitors may enhance their clinical effectiveness. Full Text Article | | 17710163
 |
Nuclear cyclin B1 in human breast carcinoma as a potent prognostic factor.
Takashi Suzuki,Tomohiro Urano,Yasuhiro Miki,Takuya Moriya,Jun-ichi Akahira,Takanori Ishida,Kuniko Horie,Satoshi Inoue,Hironobu Sasano
Cancer science
98
2007
Show Abstract
Cyclin B1 is translocated to the nucleus from the cytoplasm, and plays an essential role in cell proliferation through promotion of mitosis. Although overexpression of cyclin B1 was previously reported in breast carcinomas, the biological significance of the intracellular localization of cyclin B1 remains unclear. Therefore, in this study, we examined cyclin B1 immunoreactivity in 109 breast carcinomas, according to the intracellular localization, that is, nucleus, cytoplasm or total (nucleus or cytoplasm). Total cyclin B1 was detected in carcinoma cells in 42% of breast carcinomas examined, whereas nuclear and cytoplasmic cyclin B1 were positive in 17 and 35% of the cases, respectively. Total or cytoplasmic cyclin B1 were positively associated with histological grade, mitosis, Ki-67, p53, c-myc or 14-3-3sigma, and inversely correlated with estrogen or progesterone receptor. Nuclear cyclin B1 was significantly associated with tumor size, lymph node metastasis, histological grade, mitosis, Ki-67 or polo-like kinase 1. Only nuclear cyclin B1 was significantly associated with adverse clinical outcome of the patients, and multivariate analyses of disease-free and overall survival demonstrated nuclear cyclin B1 as the independent marker. A similar tendency was detected in the patients receiving adjuvant therapy after surgery. These results suggest that an onocogenic role of overexpressed cyclin B1 is mainly mediated in nuclei of breast carcinoma cells, and the nuclear translocation is regulated by polo-like kinase 1 and 14-3-3sigma. Nuclear cyclin B1-positive breast carcinoma is resistant to adjuvant therapy, and nuclear cyclin B1 immunoreactivity is a potent prognostic factor in breast carcinoma patients. | | 17359284
 |
Mitogenic signalling and the p16INK4a-Rb pathway cooperate to enforce irreversible cellular senescence.
Akiko Takahashi, Naoko Ohtani, Kimi Yamakoshi, Shin-ichi Iida, Hidetoshi Tahara, Keiko Nakayama, Keiichi I Nakayama, Toshinori Ide, Hideyuki Saya, Eiji Hara
Nature cell biology
8
1291-7
2005
Show Abstract
The p16(INK4a) cyclin-dependent kinase inhibitor has a key role in establishing stable G1 cell-cycle arrest through activating the retinoblastoma (Rb) tumour suppressor protein pRb in cellular senescence. Here, we show that the p16(INK4a) /Rb-pathway also cooperates with mitogenic signals to induce elevated intracellular levels of reactive oxygen species (ROS), thereby activating protein kinase Cdelta (PKCdelta) in human senescent cells. Importantly, once activated by ROS, PKCdelta promotes further generation of ROS, thus establishing a positive feedback loop to sustain ROS-PKCdelta signalling. Sustained activation of ROS-PKCdelta signalling irreversibly blocks cytokinesis, at least partly through reducing the level of WARTS (also known as LATS1), a mitotic exit network (MEN) kinase required for cytokinesis, in human senescent cells. This irreversible cytokinetic block is likely to act as a second barrier to cellular immortalization ensuring stable cell-cycle arrest in human senescent cells. These results uncover an unexpected role for the p16(INK4a)-Rb pathway and provide a new insight into how senescent cell-cycle arrest is enforced in human cells. | | 17028578
 |
Clues to CD2-associated protein involvement in cytokinesis.
Monzo, P; Gauthier, NC; Keslair, F; Loubat, A; Field, CM; Le Marchand-Brustel, Y; Cormont, M
Molecular biology of the cell
16
2891-902
2004
Show Abstract
Cytokinesis requires membrane trafficking coupled to actin remodeling and involves a number of trafficking molecules. CD2-associated protein (CD2AP) has been implicated in dynamic actin remodeling and membrane trafficking that occurs during endocytosis leading to the degradative pathway. In this study, we present several arguments for its implication in cytokinesis. First, endogenous CD2AP was found concentrated in the narrow region of the midzone microtubules during anaphase and in the midbody during late telophase. Moreover, we found that CD2AP is a membrane- and not a microtubule-associated protein. Second, the overexpression of the first two Src homology 3 domains of CD2AP, which are responsible for this localization, led to a significant increase in the rate of cell multinucleation. Third, the CD2AP small interfering RNA interfered with the cell separation, indicating that CD2AP is required for HeLa cells cytokinesis. Fourth, using the yeast two-hybrid system, we found that CD2AP interacted with anillin, a specific cleavage furrow component, and the two proteins colocalized at the midbody. Both CD2AP and anillin were found phosphorylated early in mitosis and also CD2AP phosphorylation was coupled to its delocalization from membrane to cytosol. All these observations led us to propose CD2AP as a new player in cytokinesis. Full Text Article | | 15800069
 |
Hierarchical requirement of SWI/SNF in retinoblastoma tumor suppressor-mediated repression of Plk1.
Gunawardena, RW; Siddiqui, H; Solomon, DA; Mayhew, CN; Held, J; Angus, SP; Knudsen, ES
The Journal of biological chemistry
279
29278-85
2004
Show Abstract
Plk1 (Polo-like kinase 1) is a critical regulator of cell cycle progression that harbors oncogenic activity and exhibits aberrant expression in multiple tumors. However, the mechanism through which Plk1 expression is regulated has not been extensively studied. Here we demonstrate that Plk1 is a target of the retinoblastoma tumor suppressor (RB) pathway. Activation of RB and related pocket proteins p107/p130 mediate attenuation of Plk1. Conversely, RB loss deregulates the control of Plk1 expression. RB pathway activation resulted in the repression of Plk1 promoter activity, and this action was dependent on the SWI/SNF chromatin remodeling complex. Although SWI/SNF subunits are lost during tumorigenesis and cooperate with RB for transcriptional repression, the mechanism through which SWI/SNF impinges on RB action is unresolved. Therefore, we delineated the requirement of SWI/SNF for three critical facets of Plk1 promoter regulation: transcription factor binding, corepressor binding, and histone modification. We find that E2F4 and pocket protein association with the Plk1 promoter is independent of SWI/SNF. However, these analyses revealed that SWI/SNF is required for histone deacetylation of the Plk1 promoter. The importance of SWI/SNF-dependent histone deacetylation of the Plk1 promoter was evident, because blockade of this event restored Plk1 expression in the presence of active RB. In summary, these data demonstrate that Plk1 is a target of the RB pathway. Moreover, these findings demonstrate a hierarchical role for SWI/SNF in the control of promoter activity through histone modification. | | 15105433
 |