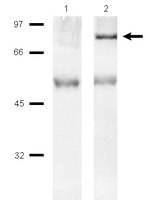
p85 Associates with unphosphorylated PTEN and the PTEN-associated complex.
Rabinovsky, R; Pochanard, P; McNear, C; Brachmann, SM; Duke-Cohan, JS; Garraway, LA; Sellers, WR
Molecular and cellular biology
29
5377-88
2009
Show Abstract
The lipid phosphatase PTEN functions as a tumor suppressor by dephosphorylating the D3 position of phosphoinositide-3,4,5-trisphosphate, thereby negatively regulating the phosphoinositide 3-kinase (PI3K)/AKT signaling pathway. In mammalian cells, PTEN exists either as a monomer or as a part of a greater than 600-kDa complex (the PTEN-associated complex [PAC]). Previous studies suggest that the antagonism of PI3K/AKT signaling by PTEN may be mediated by a nonphosphorylated form of the protein resident within the multiprotein complex. Here we show that PTEN associates with p85, the regulatory subunit of PI3K. Using newly generated antibodies, we demonstrate that this PTEN-p85 association involves the unphosphorylated form of PTEN engaged within the PAC and also includes the p110beta isoform of PI3K. The PTEN-p85 association is enhanced by trastuzumab treatment and linked to a decline in AKT phosphorylation in some ERBB2-amplified breast cancer cell lines. Together, these results suggest that integration of p85 into the PAC may provide a novel means of downregulating the PI3K/AKT pathway. | Immunoprecipitation | 19635806
 |
Calorie restriction increases the ratio of phosphatidylinositol 3-kinase catalytic to regulatory subunits in rat skeletal muscle.
McCurdy, CE; Davidson, RT; Cartee, GD
American journal of physiology. Endocrinology and metabolism
288
E996-E1001
2004
Show Abstract
Calorie restriction [CR; 60% of ad libitum (AL) intake] improves insulin-stimulated glucose transport, concomitant with enhanced phosphorylation of Akt. The mechanism(s) for the CR-induced increase in Akt phosphorylation of insulin-stimulated muscle is unknown. The purpose of this study was to determine whether CR increased the ratio of catalytic to regulatory subunits favoring enhanced phosphatidylinositol (PI) 3-kinase signaling, which may contribute to increases in Akt phosphorylation and glucose transport in insulin-stimulated muscles. We measured the PI 3-kinase regulatory (p85alpha/beta, p50alpha, and p55alpha) and catalytic (p110) subunits abundance in skeletal muscle from male F344B/N rats after 8 wk of AL or CR treatment. In CR compared with AL muscles, regulatory isoforms, p50alpha and p55alpha abundance were approximately 40% lower (P less than 0.01) with unchanged p85alpha/beta levels. There was no diet-related change in catalytic subunit abundance. Despite lower IRS-1 levels ( approximately 35%) for CR vs. AL, IRS-1-p110 association in insulin-stimulated muscles was significantly (P less than 0.05) enhanced by approximately 50%. Downstream of PI 3-kinase, CR compared with AL significantly enhanced Akt serine phosphorylation by 1.5-fold higher (P = 0.01) and 3-O-methylglucose transport by approximately 20% in muscles incubated with insulin. The increased ratio of PI 3-kinase catalytic to regulatory subunits favors enhanced insulin signaling, which likely contributes to greater Akt phosphorylation and improved insulin sensitivity associated with CR in skeletal muscle. | Immunoprecipitation | 15613677
 |