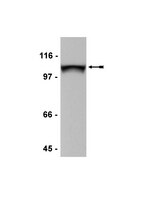
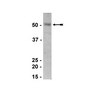
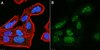
Relationship between intracellular Na+ concentration and reduced Na+ affinity in Na+,K+-ATPase mutants causing neurological disease.
Toustrup-Jensen, MS; Einholm, AP; Schack, VR; Nielsen, HN; Holm, R; Sobrido, MJ; Andersen, JP; Clausen, T; Vilsen, B
The Journal of biological chemistry
289
3186-97
2014
Show Abstract
The neurological disorders familial hemiplegic migraine type 2 (FHM2), alternating hemiplegia of childhood (AHC), and rapid-onset dystonia parkinsonism (RDP) are caused by mutations of Na(+),K(+)-ATPase α2 and α3 isoforms, expressed in glial and neuronal cells, respectively. Although these disorders are distinct, they overlap in phenotypical presentation. Two Na(+),K(+)-ATPase mutations, extending the C terminus by either 28 residues ("+28" mutation) or an extra tyrosine ("+Y"), are associated with FHM2 and RDP, respectively. We describe here functional consequences of these and other neurological disease mutations as well as an extension of the C terminus only by a single alanine. The dependence of the mutational effects on the specific α isoform in which the mutation is introduced was furthermore studied. At the cellular level we have characterized the C-terminal extension mutants and other mutants, addressing the question to what extent they cause a change of the intracellular Na(+) and K(+) concentrations ([Na(+)]i and [K(+)]i) in COS cells. C-terminal extension mutants generally showed dramatically reduced Na(+) affinity without disturbance of K(+) binding, as did other RDP mutants. No phosphorylation from ATP was observed for the +28 mutation of α2 despite a high expression level. A significant rise of [Na(+)]i and reduction of [K(+)]i was detected in cells expressing mutants with reduced Na(+) affinity and did not require a concomitant reduction of the maximal catalytic turnover rate or expression level. Moreover, two mutations that increase Na(+) affinity were found to reduce [Na(+)]i. It is concluded that the Na(+) affinity of the Na(+),K(+)-ATPase is an important determinant of [Na(+)]i. | Western Blotting | 24356962
 |
Na,K-ATPase alpha isoforms at the blood-cerebrospinal fluid-trigeminal nerve and blood-retina interfaces in the rat.
Arakaki, X; McCleary, P; Techy, M; Chiang, J; Kuo, L; Fonteh, AN; Armstrong, B; Levy, D; Harrington, MG
Fluids and barriers of the CNS
10
14
2013
Show Abstract
Cerebrospinal fluid (CSF) sodium concentration increases during migraine attacks, and both CSF and vitreous humor sodium increase in the rat migraine model. The Na,K-ATPase is a probable source of these sodium fluxes. Since Na,K-ATPase isoforms have different locations and physiological roles, our objective was to establish which alpha isoforms are present at sites where sodium homeostasis is disrupted.Specific Na,K-ATPase alpha isoforms were identified in rat tissues by immunohistochemistry at the blood-CSF barrier at the choroid plexus, at the blood-CSF-trigeminal barrier at the meninges, at the blood-retina barrier, and at the blood-aqueous barrier at the ciliary body. Calcitonin gene-related peptide (CGRP), occludin, or von Willibrand factor (vWF) were co-localized with Na,K-ATPase to identify trigeminal nociceptor fibers, tight junctions, and capillary endothelial cells respectively.The Na,K-ATPase alpha-2 isoform is located on capillaries and intensely at nociceptive trigeminal nerve fibers at the meningeal blood-CSF-trigeminal barrier. Alpha-1 and -3 are lightly expressed on the trigeminal nerve fibers but not at capillaries. Alpha-2 is expressed at the blood-retina barriers and, with alpha-1, at the ciliary body blood aqueous barrier. Intense apical membrane alpha-1 was associated with moderate cytoplasmic alpha-2 expression at the choroid plexus blood-CSF barrier.Na,K-ATPase alpha isoforms are present at the meningeal, choroid plexus, and retinal barriers. Alpha-2 predominates at the capillary endothelial cells in the meninges and retinal ganglion cell layer. | | 23497725
 |
The 10-20-30 training concept improves performance and health profile in moderately trained runners.
Gunnarsson, TP; Bangsbo, J
Journal of applied physiology (Bethesda, Md. : 1985)
113
16-24
2011
Show Abstract
The effect of an alteration from regular endurance to interval (10-20-30) training on the health profile, muscular adaptations, maximum oxygen uptake (Vo(2max)), and performance of runners was examined. Eighteen moderately trained individuals (6 females and 12 males; Vo(2max): 52.2 ± 1.5 ml·kg(-1)·min(-1)) (means ± SE) were divided into a high-intensity training (10-20-30; 3 women and 7 men) and a control (CON; 3 women and 5 men) group. For a 7-wk intervention period the 10-20-30 replaced all training sessions with 10-20-30 training consisting of low-, moderate-, and high-speed running (less than 30%, less than 60%, and greater than 90% of maximal intensity) for 30, 20, and 10 s, respectively, in three or four 5-min intervals interspersed by 2 min of recovery, reducing training volume by 54% (14.0 ± 0.9 vs. 30.4 ± 2.3 km/wk) while CON continued the normal training. After the intervention period Vo(2max) in 10-20-30 was 4% higher, and performance in a 1,500-m and a 5-km run improved (P less than 0.05) by 21 and 48 s, respectively. In 10-20-30, systolic blood pressure was reduced (P less than 0.05) by 5 ± 2 mmHg, and total and low-density lipoprotein (LDL) cholesterol was lowered (P less than 0.05) by 0.5 ± 0.2 and 0.4 ± 0.1 mmol/l, respectively. No alterations were observed in CON. Muscle membrane proteins and enzyme activity did not change in either of the groups. The present study shows that interval training with short 10-s near-maximal bouts can improve performance and Vo(2max) despite a ∼50% reduction in training volume. In addition, the 10-20-30 training regime lowers resting systolic blood pressure and blood cholesterol, suggesting a beneficial effect on the health profile of already trained individuals. | | 22556401
 |
Stabilization of the α2 isoform of Na,K-ATPase by mutations in a phospholipid binding pocket.
Kapri-Pardes, E; Katz, A; Haviv, H; Mahmmoud, Y; Ilan, M; Khalfin-Penigel, I; Carmeli, S; Yarden, O; Karlish, SJ
The Journal of biological chemistry
286
42888-99
2010
Show Abstract
The α2 isoform of Na,K-ATPase plays a crucial role in Ca(2+) handling, muscle contraction, and inotropic effects of cardiac glycosides. Thus, structural, functional, and pharmacological comparisons of α1, α2, and α3 are of great interest. In Pichia pastoris membranes expressing human α1β1, α2β1, and α3β1 isoforms, or using the purified isoform proteins, α2 is most easily inactivated by heating and detergent (α2 ≫ α3 greater than α1). We have examined an hypothesis that instability of α2 is caused by weak interactions with phosphatidylserine, which stabilizes the protein. Three residues, unique to α2, in trans-membrane segments M8 (Ala-920), M9 (Leu-955), and M10 (Val-981) were replaced by equivalent residues in α1, singly or together. Judged by the sensitivity of the purified proteins to heat, detergent, "affinity" for phosphatidylserine, and stabilization by FXYD1, the triple mutant (A920V/L955F/V981P, called α2VFP) has stability properties close to α1, although single mutants have only modest or insignificant effects. Functional differences between α1 and α2 are unaffected in α2VFP. A compound, 6-pentyl-2-pyrone, isolated from the marine fungus Trichoderma gamsii is a novel probe of specific phospholipid-protein interactions. 6-Pentyl-2-pyrone inactivates the isoforms in the order α2 ≫ α3 greater than α1, and α2VFP and FXYD1 protect the isoforms. In native rat heart sarcolemma membranes, which contain α1, α2, and α3 isoforms, a component attributable to α2 is the least stable. The data provide clear evidence for a specific phosphatidylserine binding pocket between M8, M9, and M10 and confirm that the instability of α2 is due to suboptimal interactions with phosphatidylserine. In physiological conditions, the instability of α2 may be important for its cellular regulatory functions. | | 22027833
 |
Effect of 2-wk intensified training and inactivity on muscle Na+-K+ pump expression, phospholemman (FXYD1) phosphorylation, and performance in soccer players.
Thomassen, M; Christensen, PM; Gunnarsson, TP; Nybo, L; Bangsbo, J
Journal of applied physiology (Bethesda, Md. : 1985)
108
898-905
2009
Show Abstract
The present study examined muscle adaptations and alterations in performance of highly trained soccer players with intensified training or training cessation. Eighteen elite soccer players were, for a 2-wk period, assigned to either a group that performed high-intensity training with a reduction in the amount of training (HI, n = 7), or an inactivity group without training (IN, n = 11). HI improved (P less than 0.05) performance of the 4th, 6th, and 10th sprint in a repeated 20-m sprint test, and IN reduced (P less than 0.05) performance in the 5th to the 10th sprints after the 2-wk intervention period. In addition, the Yo-Yo intermittent recovery level 2 test performance of IN was lowered from 845 +/- 48 to 654 +/- 30 m. In HI, the protein expression of the Na(+)-K(+) pump alpha(2)-isoform was 15% higher (P less than 0.05) after the intervention period, whereas no changes were observed in alpha(1)- and beta(1)-isoform expression. In IN, Na(+)-K(+) pump expression was not changed. In HI, the FXYD1ser68-to-FXYD1 ratio was 27% higher (P less than 0.01) after the intervention period, and, in IN, the AB_FXYD1ser68 signal was 18% lower (P less than 0.05) after inactivity. The change in FXYD1ser68-to-FXYD1 ratio was correlated (r(2) = 0.35; P less than 0.05) with change in performance in repeated sprint test. The present data suggest that short-term intensified training, even for trained soccer players, can increase muscle Na(+)-K(+) pump alpha(2)-isoform expression, and that cessation of training for 2 wk does not affect the expression of Na(+)-K(+) pump isoforms. Resting phosphorylation status of the Na(+)-K(+) pump is changed by training and inactivity and may play a role in performance during repeated, intense exercise. | | 20133439
 |
Reduced volume and increased training intensity elevate muscle Na+-K+ pump alpha2-subunit expression as well as short- and long-term work capacity in humans.
Bangsbo, J; Gunnarsson, TP; Wendell, J; Nybo, L; Thomassen, M
Journal of applied physiology (Bethesda, Md. : 1985)
107
1771-80
2009
Show Abstract
The present study examined muscle adaptations and alterations in work capacity in endurance-trained runners as a result of a reduced amount of training combined with speed endurance training. For a 6- to 9-wk period, 17 runners were assigned to either a speed endurance group with a 25% reduction in the amount of training but including speed endurance training consisting of six to twelve 30-s sprint runs 3-4 times/wk (SET group n = 12) or a control group (n = 5), which continued the endurance training ( approximately 55 km/wk). For the SET group, the expression of the muscle Na(+)-K(+) pump alpha(2)-subunit was 68% higher (P less than 0.05) and the plasma K(+) level was reduced (P less than 0.05) during repeated intense running after 9 wk. Performance in a 30-s sprint test and the first of the supramaximal exhaustive runs was improved (P less than 0.05) by 7% and 36%, respectively, after the speed endurance training period. In the SET group, maximal O(2) uptake was unaltered, but the 3-km (3,000-m) time was reduced (P less than 0.05) from 10.4 +/- 0.1 to 10.1 +/- 0.1 min and the 10-km (10,000-m) time was improved from 37.3 +/- 0.4 to 36.3 +/- 0.4 min (means +/- SE). Muscle protein expression and performance remained unaltered in the control group. The present data suggest that both short- and long-term exercise performances can be improved with a reduction in training volume if speed endurance training is performed and that the Na(+)-K(+) pump plays a role in the control of K(+) homeostasis and in the development of fatigue during repeated high-intensity exercise. | | 19797693
 |
Modulation of endothelial cell KCa3.1 channels during endothelium-derived hyperpolarizing factor signaling in mesenteric resistance arteries.
Dora, KA; Gallagher, NT; McNeish, A; Garland, CJ
Circulation research
102
1247-55
2008
Show Abstract
Arterial hyperpolarization to acetylcholine (ACh) reflects coactivation of K(Ca)3.1 (IK(Ca)) channels and K(Ca)2.3 (SK(Ca)) channels in the endothelium that transfers through myoendothelial gap junctions and diffusible factor(s) to affect smooth muscle relaxation (endothelium-derived hyperpolarizing factor [EDHF] response). However, ACh can differentially activate K(Ca)3.1 and K(Ca)2.3 channels, and we investigated the mechanisms responsible in rat mesenteric arteries. K(Ca)3.1 channel input to EDHF hyperpolarization was enhanced by reducing external [Ca(2+)](o) but blocked either with forskolin to activate protein kinase A or by limiting smooth muscle [Ca(2+)](i) increases stimulated by phenylephrine depolarization. Imaging [Ca(2+)](i) within the endothelial cell projections forming myoendothelial gap junctions revealed increases in cytoplasmic [Ca(2+)](i) during endothelial stimulation with ACh that were unaffected by simultaneous increases in muscle [Ca(2+)](i) evoked by phenylephrine. If gap junctions were uncoupled, K(Ca)3.1 channels became the predominant input to EDHF hyperpolarization, and relaxation was inhibited with ouabain, implicating a crucial link through Na(+)/K(+)-ATPase. There was no evidence for an equivalent link through K(Ca)2.3 channels nor between these channels and the putative EDHF pathway involving natriuretic peptide receptor-C. Reconstruction of confocal z-stack images from pressurized arteries revealed K(Ca)2.3 immunostain at endothelial cell borders, including endothelial cell projections, whereas K(Ca)3.1 channels and Na(+)/K(+)-ATPase alpha(2)/alpha(3) subunits were highly concentrated in endothelial cell projections and adjacent to myoendothelial gap junctions. Thus, extracellular [Ca(2+)](o) appears to modify K(Ca)3.1 channel activity through a protein kinase A-dependent mechanism independent of changes in endothelial [Ca(2+)](i). The resulting hyperpolarization links to arterial relaxation largely through Na(+)/K(+)-ATPase, possibly reflecting K(+) acting as an EDHF. In contrast, K(Ca)2.3 hyperpolarization appears mainly to affect relaxation through myoendothelial gap junctions. Overall, these data suggest that K(+) and myoendothelial coupling evoke EDHF-mediated relaxation through distinct, definable pathways. | | 18403729
 |
The Na+/K+-ATPase alpha2-isoform regulates cardiac contractility in rat cardiomyocytes.
Swift, F; Tovsrud, N; Enger, UH; Sjaastad, I; Sejersted, OM
Cardiovascular research
75
109-17
2007
Show Abstract
The presence of both alpha1- and alpha2-isoforms of the Na+/K+-ATPase (NKA) in cardiomyocytes indicates different functions. We hypothesized that preferential localization of the alpha2-isoform to the t-tubules, locally controlling the Na+/Ca2+-exchanger (NCX), underlies a specific role in Ca2+ handling.We studied NKA isoform distribution in isolated cardiomyocytes from Wistar rats using immunocytochemistry. NKA pump and NCX currents (I(pump) and I(NCX)) were measured in control and detubulated cardiomyocytes. Intracellular Na+ concentration [Na+]i was assessed with the fluorescent dye SBFI.The alpha2-isoform abundance was higher in the t-tubules than in the surface sarcolemma. We established that 0.3 microM ouabain specifically blocked the alpha2-isoform in isolated rat cardiomyocytes. This low concentration blocked 10.7+/-0.6% of I(pump) in control, but only 6.0+/-0.5% in detubulated cardiomyocytes. Moreover, measured and calculated alpha1-specific and alpha2-specific I(pump) in control (547+/-29 pA and 66 pA, respectively) and in detubulated cells (495+/-30 pA and 31 pA, respectively) showed that 53% of the alpha2-isoform, but only 9.5% of the alpha1-isoform, were localized to the t-tubules. Despite the small abundance of the alpha2-isoform (approximately 11% of total NKA), selective inhibition of this isoform induced a 40% increase in contractility in field stimulated cardiomyocytes, but no increase in global [Na+]i. However, inhibition of the alpha2-isoform increased I(NCX) indicating local subsarcolemmal accumulation of Na+ near NCX.The alpha2-isoform of the NKA is functionally coupled to the NCX and can regulate Ca2+ handling without changing global [Na+]i. | Western Blotting | 17442282
 |
The molecular genetics of migraine.
Wessman, Maija, et al.
Ann. Med., 36: 462-73 (2004)
2004
Show Abstract
Within the past decade it has been possible to identify susceptibility gene loci that predispose to migraine using genetic markers distributed across the human genome. Five new loci with significant linkage to common types of migraine--migraine with or without aura--have been identified on four different chromosomes using a genome-wide screen approach. So far, only the locus on 4q has been replicated but no specific, disease-causing mutations have been described in these common forms of migraine. The best genetic evidence providing molecular insight into migraine still comes from the mutations detected in a rare Mendelian form of migraine with aura--familial hemiplegic migraine (FHM). In 50%-70% of FHM families, mutations in the calcium channel gene CACNA1A in chromosome 19p13 have been identified. In some families, mutations in the ATP1A2 gene encoding the alpha2 subunit of the Na+, K+-ATPase are associated with FHM, linked to 1q23. Here we discuss the current knowledge of the heritability of migraine and rare migraine variants as models for understanding the pathophysiology of common migraine and animal models that might contribute to understanding common forms of migraine. | | 15513297
 |
The Na, K-ATPase in the failing human heart.
Schwinger, Robert H G, et al.
Cardiovasc. Res., 57: 913-20 (2003)
2003
Show Abstract
The Na, K-ATPase consists of alpha- and beta-subunits and actively transports Na out and K into the myocyte. It is the receptor for cardiac glycosides exerting its positive inotropic effect by inhibiting enzyme activity, decreasing the driving force for the Na/Ca-exchange and increasing cellular content and release of Ca during depolarization. The specific binding capacity for cardiac glycosides is utilized as a tool for Na, K-ATPase quantification with high accuracy and precision. In treatment of patients with heart failure cardiac glycosides improve symptoms and reduce the need for hospitalization without affecting mortality. In endomyocardial biopsies from patients with compromised cardiac function total Na, K-ATPase concentration is decreased by approximately 40% and a correlation between decrease in heart function and decrease in Na, K-ATPase concentration exists. At the subunit level, the alpha1-, alpha3- and beta1-proteins are reduced in human heart failure. During digitalization approximately 30% of remaining Na, K-pumps are occupied by digoxin. Thus, a total of not less than half the Na, K-pumps may be out of function in the myocardium of digitalised heart failure patients. It is still a matter of debate whether a digitalis-like factor exists. There is a pressing need for the identification of its precise chemical structure, properties and quantitative relation to the Na, K-ATPase. It is recommended that cardiac glycosides are prescribed to heart failure patients who are still having heart failure symptoms after institution of mortality reducing therapy. Cardiac glycoside treatment is still the only safe inotropic drug for oral use that improves hemodynamics in patients with compromised cardiac function. | | 12650869
 |