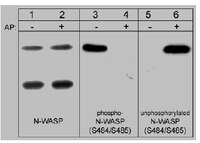
Estrogen receptor-alpha promotes breast cancer cell motility and invasion via focal adhesion kinase and N-WASP.
Sanchez, AM; Flamini, MI; Baldacci, C; Goglia, L; Genazzani, AR; Simoncini, T
Molecular endocrinology (Baltimore, Md.)
24
2114-25
2009
Show Abstract
The ability of cancer cells to move and invade the surrounding environment is the basis of local and distant metastasis. Cancer cell movement requires dynamic remodeling of the cytoskeleton and cell membrane and is controlled by multiprotein complexes including focal adhesion kinase (FAK) or the Neural Wiskott-Aldrich Syndrome Protein (N-WASP). We show that 17β-estradiol induces phosphorylation of FAK and its translocation toward membrane sites where focal adhesion complexes are assembled. This process is triggered via a Gα/Gβ protein-dependent, rapid extranuclear signaling of estrogen receptor α interacts in a multiprotein complex with c-Src, phosphatidylinositol 3-OH kinase, and FAK. Within this complex FAK autophosphorylation ensues, and activated FAK recruits the small GTPase cdc42, which, in turn, triggers N-WASP phosphorylation. This results in the translocation of Arp2/3 complexes at sites where membrane structures related to cell movement are formed. Recruitment of FAK and N-WASP is necessary for cell migration and invasion induced by 17β-estradiol in breast cancer cells. Our findings identify an original mechanism through which estrogen promotes breast cancer cell motility and invasion. This information helps to understand the effects of estrogen on breast cancer metastasis and may provide new targets for therapeutic interventions. | 20880986
 |