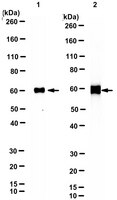
Homing of neural stem cells from the venous compartment into a brain infarct does not involve conventional interactions with vascular endothelium.
Goncharova, V; Das, S; Niles, W; Schraufstatter, I; Wong, AK; Povaly, T; Wakeman, D; Miller, L; Snyder, EY; Khaldoyanidi, SK
Stem cells translational medicine
3
229-40
2014
Show Abstract
Human neural stem cells (hNSCs) hold great potential for treatment of a wide variety of neurodegenerative and neurotraumatic conditions. Heretofore, administration has been through intracranial injection or implantation of cells. Because neural stem cells are capable of migrating to the injured brain from the intravascular space, it seemed feasible to administer them intravenously if their ability to circumvent the blood-brain barrier was enhanced. In the present studies, we found that interactions of hNSCs in vitro on the luminal surface of human umbilical vein endothelial cells was enhanced following enforced expression of cutaneous lymphocyte antigen on cell surface moieties by incubation of hNSCs with fucosyltransferase VI and GDP-fucose (fhNSCs). Interestingly, ex vivo fucosylation of hNSCs not only did not improve the cells homing into the brain injured by stroke following intravenous administration but also increased mortality of rats compared with the nonfucosylated hNSC group. Efforts to explain these unexpected findings using a three-dimensional flow chamber device revealed that transmigration of fhNSCs (under conditions of physiological shear stress) mediated by stromal cell-derived factor 1α was significantly decreased compared with controls. Further analysis revealed that hNSCs poorly withstand physiological shear stress, and their ability is further decreased following fucosylation. In addition, fhNSCs demonstrated a higher frequency of cellular aggregate formation as well as a tendency for removal of fucose from the cell surface. In summary, our findings suggest that the behavior of hNSCs in circulation is different from that observed with other cell types and that, at least for stroke, intravenous administration is a suboptimal route, even when the in vitro rolling ability of hNSCs is optimized by enforced fucosylation. | | | 24396034
 |
A Site-Specific Integrated Col2.3GFP Reporter Identifies Osteoblasts Within Mineralized Tissue Formed In Vivo by Human Embryonic Stem Cells.
Xin, X; Jiang, X; Wang, L; Stover, ML; Zhan, S; Huang, J; Goldberg, AJ; Liu, Y; Kuhn, L; Reichenberger, EJ; Rowe, DW; Lichtler, AC
Stem cells translational medicine
3
1125-37
2014
Show Abstract
The use of human embryonic stem cells (hESCs) and induced pluripotent stem cells (iPSCs) for study and treatment of bone diseases or traumatic bone injuries requires efficient protocols to differentiate hESCs/iPSCs into cells with osteogenic potential and the ability to isolate differentiated osteoblasts for analysis. We have used zinc finger nuclease technology to deliver a construct containing the Col2.3 promoter driving GFPemerald to the AAVS1 site (referred to as a "safe harbor" site), in human embryonic stem cells (H9Zn2.3GFP), with the goal of marking the cells that have become differentiated osteoblasts. In teratomas formed using these cells, we identified green fluorescent protein (GFP)-positive cells specifically associated with in vivo bone formation. We also differentiated the cells into a mesenchymal stem cell population with osteogenic potential and implanted them into a mouse calvarial defect model. We observed GFP-positive cells associated with alizarin complexone-labeled newly formed bone surfaces. The cells were alkaline phosphatase-positive, and immunohistochemistry with human specific bone sialoprotein (BSP) antibody indicates that the GFP-positive cells are also associated with the human BSP-containing matrix, demonstrating that the Col2.3GFP construct marks cells in the osteoblast lineage. Single-cell cloning generated a 100% Col2.3GFP-positive cell population, as demonstrated by fluorescence in situ hybridization using a GFP probe. The karyotype was normal, and pluripotency was demonstrated by Tra1-60 immunostaining, pluripotent low density reverse transcription-polymerase chain reaction array and embryoid body formation. These cells will be useful to develop optimal osteogenic differentiation protocols and to isolate osteoblasts from normal and diseased iPSCs for analysis. | | | 25122686
 |
Role of mesenchymal stem cells in osteosarcoma and metabolic reprogramming of tumor cells.
Bonuccelli, G; Avnet, S; Grisendi, G; Salerno, M; Granchi, D; Dominici, M; Kusuzaki, K; Baldini, N
Oncotarget
5
7575-88
2014
Show Abstract
The tumor microenvironment plays an important role in cancer progression. Here, we focused on the role of reactive mesenchymal stem cells (MSC) in osteosarcoma (OS), and used human adipose MSC and a panel of OS cell lines (Saos-2, HOS, and 143B) to investigate the mutual effect of normal-cancer cell metabolic programming. Our results showed that MSC are driven by oxidative stress induced by OS cells to undergo Warburg metabolism, with increased lactate production. Therefore, we analyzed the expression of lactate monocarboxylate transporters. By real time PCR and immunofluorescence, in MSC we detected the expression of MCT-4, the transporter for lactate efflux, whereas MCT-1, responsible for lactate uptake, was expressed in OS cells. In agreement, silencing of MCT-1 by siRNA significantly affected the ATP production in OS cancer cells. Thus, cancer cells directly increase their mitochondrial biogenesis using this energy-rich metabolite that is abundantly provided by MSC as an effect of the altered microenvironmental conditions induced by OS cells. We also showed that lactate produced by MSC promotes the migratory ability of OS cells. These data provide novel information to be exploited for cancer therapies targeting the mutual metabolic reprogramming of cancer cells and their stroma. | | | 25277190
 |
Developmental-like bone regeneration by human embryonic stem cell-derived mesenchymal cells.
Kuhn, LT; Liu, Y; Boyd, NL; Dennis, JE; Jiang, X; Xin, X; Charles, LF; Wang, L; Aguila, HL; Rowe, DW; Lichtler, AC; Goldberg, AJ
Tissue engineering. Part A
20
365-77
2014
Show Abstract
The in vivo osteogenesis potential of mesenchymal-like cells derived from human embryonic stem cells (hESC-MCs) was evaluated in vivo by implantation on collagen/hydroxyapatite scaffolds into calvarial defects in immunodeficient mice. This study is novel because no osteogenic or chondrogenic differentiation protocols were applied to the cells prior to implantation. After 6 weeks, X-ray, microCT, and histological analysis showed that the hESC-MCs had consistently formed a highly vascularized new bone that bridged the bone defect and seamlessly integrated with host bone. The implanted hESC-MCs differentiated in situ to functional hypertrophic chondrocytes, osteoblasts, and osteocytes forming new bone tissue via an endochondral ossification pathway. Evidence for the direct participation of the human cells in bone morphogenesis was verified by two separate assays: with Alu and by human mitochondrial antigen positive staining in conjunction with co-localized expression of human bone sialoprotein in histologically verified regions of new bone. The large volume of new bone in a calvarial defect and the direct participation of the hESC-MCs far exceeds that of previous studies and that of the control adult hMSCs. This study represents a key step forward for bone tissue engineering because of the large volume, vascularity, and reproducibility of new bone formation and the discovery that it is advantageous to not over-commit these progenitor cells to a particular lineage prior to implantation. The hESC-MCs were able to recapitulate the mesenchymal developmental pathway and were able to repair the bone defect semi-autonomously without preimplantation differentiation to osteo- or chondroprogenitors. | | | 23952622
 |
Neural progenitors derived from human induced pluripotent stem cells survive and differentiate upon transplantation into a rat model of amyotrophic lateral sclerosis.
Popescu, IR; Nicaise, C; Liu, S; Bisch, G; Knippenberg, S; Daubie, V; Bohl, D; Pochet, R
Stem cells translational medicine
2
167-74
2013
Show Abstract
Human induced pluripotent stem cells (iPSCs) offer hope for personalized regenerative cell therapy in amyotrophic lateral sclerosis (ALS). We analyzed the fate of human iPSC-derived neural progenitors transplanted into the spinal cord of wild-type and transgenic rats carrying a human mutated SOD1(G93A) gene. The aim was to follow survival and differentiation of human neural progenitors until day 60 post-transplantation in two different in vivo environments, one being ALS-like. iPSC-derived neural progenitors efficiently engrafted in the adult spinal cord and survived at high numbers. Different neural progenitor, astroglial, and neuronal markers indicated that, over time, the transplanted nestin-positive cells differentiated into cells displaying a neuronal phenotype in both wild-type and transgenic SOD1 rats. Although a transient microglial phenotype was detected at day 15, astroglial staining was negative in engrafted cells from day 1 to day 60. At day 30, differentiation toward a neuronal phenotype was identified, which was further established at day 60 by the expression of the neuronal marker MAP2. A specification process into motoneuron-like structures was evidenced in the ventral horns in both wild-type and SOD1 rats. Our results demonstrate proof-of-principle of survival and differentiation of human iPSC-derived neural progenitors in in vivo ALS environment, offering perspectives for the use of iPSC-based therapy in ALS. | | | 23413376
 |
Platelet-rich plasma promotes the proliferation of human muscle derived progenitor cells and maintains their stemness.
Li, H; Usas, A; Poddar, M; Chen, CW; Thompson, S; Ahani, B; Cummins, J; Lavasani, M; Huard, J
PloS one
8
e64923
2013
Show Abstract
Human muscle-derived progenitor cells (hMDPCs) offer great promise for muscle cell-based regenerative medicine; however, prolonged ex-vivo expansion using animal sera is necessary to acquire sufficient cells for transplantation. Due to the risks associated with the use of animal sera, the development of a strategy for the ex vivo expansion of hMDPCs is required. The purpose of this study was to investigate the efficacy of using platelet-rich plasma (PRP) for the ex-vivo expansion of hMDPCs. Pre-plated MDPCs, myoendothelial cells, and pericytes are three populations of hMDPCs that we isolated by the modified pre-plate technique and Fluorescence Activated Cell Sorting (FACS), respectively. Pooled allogeneic human PRP was obtained from a local blood bank, and the effect that thrombin-activated PRP-releasate supplemented media had on the ex-vivo expansion of the hMDPCs was tested against FBS supplemented media, both in vitro and in vivo. PRP significantly enhanced short and long-term cell proliferation, with or without FBS supplementation. Antibody-neutralization of PDGF significantly blocked the mitogenic/proliferative effects that PRP had on the hMDPCs. A more stable and sustained expression of markers associated with stemness, and a decreased expression of lineage specific markers was observed in the PRP-expanded cells when compared with the FBS-expanded cells. The in vitro osteogenic, chondrogenic, and myogenic differentiation capacities of the hMDPCs were not altered when expanded in media supplemented with PRP. All populations of hMDPCs that were expanded in PRP supplemented media retained their ability to regenerate myofibers in vivo. Our data demonstrated that PRP promoted the proliferation and maintained the multi-differentiation capacities of the hMDPCs during ex-vivo expansion by maintaining the cells in an undifferentiated state. Moreover, PDGF appears to be a key contributing factor to the beneficial effect that PRP has on the proliferation of hMDPCs. | | | 23762264
 |
Origin of the vasculature supporting growth of primary patient tumor xenografts.
Hylander, BL; Punt, N; Tang, H; Hillman, J; Vaughan, M; Bshara, W; Pitoniak, R; Repasky, EA
Journal of translational medicine
11
110
2013
Show Abstract
Studies of primary patient tumor xenografts grown in immunodeficient mice have shown that these tumors histologically and genetically closely resemble the original tumors. These patient xenograft models are becoming widely used for therapeutic efficacy studies. Because many therapies are directed at tumor stromal components and because the tumor microenvironment also is known to influence the response of a tumor to therapy, it is important to understand the nature of the stroma and, in particular, the vascular supply of patient xenografts.Patient tumor xenografts were established by implanting undisrupted pieces of patient tumors in SCID mice. For this study, formalin fixed, paraffin embedded specimens from several types of solid tumors were selected and, using species-specific antibodies which react with formalin fixed antigens, we analyzed the species origin of the stroma and blood vessels that supported tumor growth in these models. Additionally, we investigated the kinetics of the vascularization process in a colon tumor and a mesothelioma xenograft. In mice bearing a head and neck xenograft, a perfusion study was performed to compare the functionality of the human and mouse tumor vessels.In patient tumors which successfully engrafted, the human stroma and vessels which were engrafted as part of the original tumor did not survive and were no longer detectable at the time of first passage (15-25 weeks). Uniformly, the stroma and vessels supporting the growth of these tumors were of murine origin. The results of the kinetic studies showed that the loss of the human vessels and vascularization by host vessels occurred more rapidly in a colon tumor (by 3 weeks) than in a mesothelioma (by 9 weeks). Finally, the perfusion studies revealed that while mouse vessels in the periphery of the tumor were perfused, those in the central regions were rarely perfused. No vessels of human origin were detected in this model.In the tumors we investigated, we found no evidence that the human stromal cells and vessels contained in the original implant either survived or contributed in any substantive way to the growth of these xenografts. | | | 23639003
 |
Functional transplantation of salivary gland cells differentiated from mouse early ES cells in vitro.
Kawakami, M; Ishikawa, H; Tachibana, T; Tanaka, A; Mataga, I
Human cell
26
80-90
2013
Show Abstract
Atrophy or hypofunction of the salivary gland because of aging or disease causes hyposalivation and has an effect on the quality of life of patients, for example not only dry mouth but deterioration in mastication/deglutition disorder and the status of oral hygiene. Currently conducted therapies for atrophy or hypofunction of the salivary gland in clinical practice are only symptomatic treatments with drugs and artificial saliva, and therefore it is preferable to establish a radical therapy. At this time, as a fundamental investigation, by co-culturing mouse early ES (mEES-6) cells with human salivary gland-derived fibroblasts (hSG-fibro), differentiation of mEES-6 cells to salivary gland cells has been attempted. Also, the possibility of cell engraftment was examined. After identifying the cells which were co-cultured with GFP-transfected mEES-6 cells and hSG-fibro, the cells were transplanted into the submandibular gland of SCID mice, and the degree of differentiation into tissues was examined. The possibility of tissue functional reconstitution from co-cultured cells in a three-dimensional culture system was examined. Our results confirmed that the co-cultured cells expressed salivary gland-related markers and had an ability to generate neo-tissues by transplantation in vivo. Moreover, the cells could reconstitute gland structures in a three-dimensional culture system. By co-culture with hSG-fibro, mEES-6 cells were successfully differentiated into salivary gland cells which were transplantable and have tissue neogenetic ability. | | | 23681939
 |
Magnetic resonance imaging tracking of ferumoxytol-labeled human neural stem cells: studies leading to clinical use.
Gutova, M; Frank, JA; D'Apuzzo, M; Khankaldyyan, V; Gilchrist, MM; Annala, AJ; Metz, MZ; Abramyants, Y; Herrmann, KA; Ghoda, LY; Najbauer, J; Brown, CE; Blanchard, MS; Lesniak, MS; Kim, SU; Barish, ME; Aboody, KS; Moats, RA
Stem cells translational medicine
2
766-75
2013
Show Abstract
Numerous stem cell-based therapies are currently under clinical investigation, including the use of neural stem cells (NSCs) as delivery vehicles to target therapeutic agents to invasive brain tumors. The ability to monitor the time course, migration, and distribution of stem cells following transplantation into patients would provide critical information for optimizing treatment regimens. No effective cell-tracking methodology has yet garnered clinical acceptance. A highly promising noninvasive method for monitoring NSCs and potentially other cell types in vivo involves preloading them with ultrasmall superparamagnetic iron oxide nanoparticles (USPIOs) to enable cell tracking using magnetic resonance imaging (MRI). We report here the preclinical studies that led to U.S. Food and Drug Administration approval for first-in-human investigational use of ferumoxytol to label NSCs prior to transplantation into brain tumor patients, followed by surveillance serial MRI. A combination of heparin, protamine sulfate, and ferumoxytol (HPF) was used to label the NSCs. HPF labeling did not affect cell viability, growth kinetics, or tumor tropism in vitro, and it enabled MRI visualization of NSC distribution within orthotopic glioma xenografts. MRI revealed dynamic in vivo NSC distribution at multiple time points following intracerebral or intravenous injection into glioma-bearing mice that correlated with histological analysis. Preclinical safety/toxicity studies of intracerebrally administered HPF-labeled NSCs in mice were also performed, and they showed no significant clinical or behavioral changes, no neuronal or systemic toxicities, and no abnormal accumulation of iron in the liver or spleen. These studies support the clinical use of ferumoxytol labeling of cells for post-transplant MRI visualization and tracking. | | | 24014682
 |
Human prostate cancer in a clinically relevant xenograft mouse model: identification of β(1,6)-branched oligosaccharides as a marker of tumor progression.
Lange, T; Ullrich, S; Müller, I; Nentwich, MF; Stübke, K; Feldhaus, S; Knies, C; Hellwinkel, OJ; Vessella, RL; Abramjuk, C; Anders, M; Schröder-Schwarz, J; Schlomm, T; Huland, H; Sauter, G; Schumacher, U
Clinical cancer research : an official journal of the American Association for Cancer Research
18
1364-73
2011
Show Abstract
To establish xenograft mouse models of metastatic and nonmetastatic human prostate cancer and to apply these models to the search for aberrant glycosylation patterns associated with tumor progression in vivo and in patients.Prostate cancer cells (LNCaP, PC-3, LuCaP 23.1, and DU-145) were xenografted subcutaneously into immunodeficient pfp(-/-)/rag2(-/-) mice. Tumor growth and metastasis formation were quantified and as altered glycosylation patterns have been associated with metastasis formation in several other malignancies, prostate cancer cells were profiled by a quantitative real-time PCR (qRT-PCR) glycosylation array and compared with normal human prostate cells. The activity of upregulated glycosyltransferases was analyzed by their sugar residues end products using lectin histochemistry on primary tumors and metastases in the animal experiments and on 2,085 clinical samples.PC-3 cells produced the largest number of spontaneous lung metastases, followed by LNCaP and LuCaP 23.1, whereas DU-145 was nonmetastatic. qRT-PCR revealed an upregulation of β1,6-N-acetylglucosaminyltransferase-5b (Mgat5b) in all prostate cancer cell lines. Mgat5b products [β(1,6)-branched oligosaccharides] were predominantly detectable in metastatic xenografts as shown by increased binding of Phaseolus vulgaris leukoagglutinin (PHA-L). The percentage of prostate cancer patients who were PHA-L positive was 86.5. PHA-L intensity correlated with serum prostate-specific antigen and a cytoplasmic staining negatively affected disease-free survival.We show a novel xenograft mouse model for human prostate cancer respecting the complete metastatic cascade. Specific glycosylation patterns reveal Mgat5b products as relevant markers of both metastatic competence in mice and disease-free survival in patients. This is the first description of Mgat5b in prostate cancer indicating a significant biologic importance of β(1,6)-branched oligosaccharides for prostate cancer progression. | | | 22261809
 |