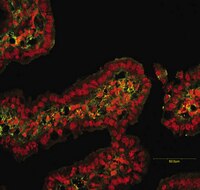
HPV16 oncoproteins induce MMPs/RECK-TIMP-2 imbalance in primary keratinocytes: possible implications in cervical carcinogenesis.
Cardeal, LB; Boccardo, E; Termini, L; Rabachini, T; Andreoli, MA; di Loreto, C; Longatto Filho, A; Villa, LL; Maria-Engler, SS
PloS one
7
e33585
2011
Show Abstract
Cervical cancer is the third most common cancer in women worldwide. Persistent infection with high-risk HPV types, principally HPV16 and 18 is the main risk factor for the development of this malignancy. However, the onset of invasive tumor occurs many years after initial exposure in a minority of infected women. This suggests that other factors beyond viral infection are necessary for tumor establishment and progression. Tumor progression is characterized by an increase in secretion and activation of matrix metalloproteinases (MMPs) produced by either the tumor cells themselves or tumor-associated fibroblasts or macrophages. Increased MMPs expression, including MMP-2, MMP-9 and MT1-MMP, has been observed during cervical carcinoma progression. These proteins have been associated with degradation of ECM components, tumor invasion, metastasis and recurrence. However, few studies have evaluated the interplay between HPV infection and the expression and activity of MMPs and their regulators in cervical cancer. We analyzed the effect of HPV16 oncoproteins on the expression and activity of MMP-2, MMP-9, MT1-MMP, and their inhibitors TIMP-2 and RECK in cultures of human keratinocytes. We observed that E7 expression is associated with increased pro-MMP-9 activity in the epithelial component of organotypic cultures, while E6 and E7 oncoproteins co-expression down-regulates RECK and TIMP-2 levels in organotypic and monolayers cultures. Finally, a study conducted in human cervical tissues showed a decrease in RECK expression levels in precancer and cancer lesions. Our results indicate that HPV oncoproteins promote MMPs/RECK-TIMP-2 imbalance which may be involved in HPV-associated lesions outcome. | 22438955
 |
Tumor-associated macrophages provide a suitable microenvironment for non-small lung cancer invasion and progression.
Rui Wang,Jie Zhang,Sufeng Chen,Meng Lu,Xiaoyang Luo,Shihua Yao,Shilei Liu,Ying Qin,Haiquan Chen
Lung cancer (Amsterdam, Netherlands)
74
2010
Show Abstract
It remains largely unknown whether tumor-associated macrophages (TAMs) are involved in invasion and metastasis of human lung cancer. The aim of our study was to obtain an accurate overview of the broad range of changes occurring in monocytes that develop into TAMs, and the roles of TAMs during the progression of non-small cell lung cancer. | 21601305
 |
Matrix metalloproteinase-9 is upregulated in nucleophosmin-anaplastic lymphoma kinase-positive anaplastic lymphomas and activated at the cell surface by the chaperone heat shock protein 90 to promote cell invasion.
Lagarrigue, F; Dupuis-Coronas, S; Ramel, D; Delsol, G; Tronchère, H; Payrastre, B; Gaits-Iacovoni, F
Cancer research
70
6978-87
2009
Show Abstract
Many anaplastic large cell lymphomas (ALCL) express the chimeric oncogene NPM-ALK, which drives malignant transformation and invasion. In this study, we show that NPM-ALK expression increases matrix metalloproteinase-9 (MMP-9) expression. Accordingly, we found that 100% of a large panel of ALK(+) ALCL biopsies examined were also MMP-9(+), in contrast to only 36.3% of ALK(-) tumors. Mechanistic studies revealed that Rac1 drove MMP-9 secretion. The MMP inhibitor GM6001 and MMP-9 blocking antibodies abolished the invasiveness of NPM-ALK(+) cells. Interestingly, the hyaluronan receptor CD44 acted as a docking surface for MMP-9 and the chaperone heat shock protein 90 on the cell surface, where MMP-9 was cleaved and activated. Membrane-associated MMP-9 was localized to invadopodia, which display a strong gelatinase activity. Taken together, our observations strengthen the concept that chaperones have a major extracellular role in the regulation of protein activation status, and reveal new factors that are crucial for spreading and invasion of ALK(+) ALCL. They also point out new factors crucial for ALK(+) ALCL. | 20699364
 |
Macrophage fusion, giant cell formation, and the foreign body response require matrix metalloproteinase 9.
MacLauchlan, S; Skokos, EA; Meznarich, N; Zhu, DH; Raoof, S; Shipley, JM; Senior, RM; Bornstein, P; Kyriakides, TR
Journal of leukocyte biology
85
617-26
2009
Show Abstract
Macrophages undergo fusion to form multinucleated giant cells in several pathologic conditions, including the foreign body response (FBR). We detected high levels of matrix metalloproteinase (MMP)-9 during macrophage fusion in vitro and in foreign body giant cells (FBGCs) in vivo. Wild-type (WT) bone marrow-derived macrophages were induced to fuse with IL-4 in the presence of MMP-9 function-blocking antibodies and displayed reduced fusion. A similar defect, characterized by delayed shape change and abnormal morphology, was observed in MMP-9 null macrophages. Analysis of the FBR in MMP-9 null mice was then pursued to evaluate the significance of these findings. Specifically, mixed cellulose ester disks and polyvinyl alcohol sponges were implanted s.c. in MMP-9 null and WT mice and excised 2-4 weeks later. Histochemical and immunohistochemical analyses indicated equal macrophage recruitment between MMP-9 null and WT mice, but FBGC formation was compromised in the former. In addition, MMP-9 null mice displayed abnormalities in extracellular matrix assembly and angiogenesis. Consistent with a requirement for MMP-9 in fusion, we also observed reduced MMP-9 levels in MCP-1 null macrophages, previously shown to be defective in FBGC formation. Collectively, our studies show abnormalities in MMP-9 null mice during the FBR and suggest a role for MMP-9 in macrophage fusion. | 19141565
 |
Mice that lack matrix metalloproteinase-9 display delayed wound healing associated with delayed reepithelization and disordered collagen fibrillogenesis.
Kyriakides, TR; Wulsin, D; Skokos, EA; Fleckman, P; Pirrone, A; Shipley, JM; Senior, RM; Bornstein, P
Matrix biology : journal of the International Society for Matrix Biology
28
65-73
2009
Show Abstract
Matrix metalloproteinase- (MMP-9) is involved in processes that occur during cutaneous wound healing such as inflammation, matrix remodeling, and epithelialization, To investigate its role in healing, full thickness skin wounds were made in the dorsal region of MMP-9-null and control mice and harvested up to 14 days post wounding. Gross examination and histological and immunohistochemical analysis indicated delayed healing in MMP-9-null mice. Specifically, MMP-9-null wounds displayed compromised reepithelialization and reduced clearance of fibrin clots. In addition, they exhibited abnormal matrix deposition, as evidenced by the irregular alignment of immature collagen fibers. Despite the presence of matrix abnormalities, MMP-9-null wounds displayed normal tensile strength. Ultrastructural analysis of wounds revealed the presence of large collagen fibrils, some with irregular shape. Keratinocyte proliferation, inflammation, and angiogenesis were found to be normal in MMP-9-null wounds. In addition, VEGF levels were similar in control and MMP-9-null wound extracts. To investigate the importance of MMP-9 in wound reepithelialization we tested human and murine keratinocytes in a wound migration assay and found that antibody-based blockade of MMP-9 function or MMP-9 deficiency retarded migration. Collectively, our observations reveal defective healing in MMP-9-null mice and suggest that MMP-9 is required for normal progression of wound closure. | 19379668
 |
Tumor-specific urinary matrix metalloproteinase fingerprinting: identification of high molecular weight urinary matrix metalloproteinase species.
Roy, R; Louis, G; Loughlin, KR; Wiederschain, D; Kilroy, SM; Lamb, CC; Zurakowski, D; Moses, MA
Clinical cancer research : an official journal of the American Association for Cancer Research
14
6610-7
2008
Show Abstract
We have previously reported that matrix metalloproteinases MMP-2, MMP-9, and the complex MMP-9/NGAL can be detected in urine of patients with a variety of cancers including prostate and bladder carcinoma. In addition, we also detected several unidentified urinary gelatinase activities with molecular weights greater than 125 kDa. The objective of the current study was to identify these high molecular weight (HMW) species, determine their potential as predictors of disease status, and ask whether a tumor-specific pattern existed based on urinary MMP analysis.Chromatography, zymography, and mass spectrometry was used to identify HMW gelatinase species of approximately 140, 190, and greater than 220 kDa in urine of cancer patients. To determine whether a tumor-specific pattern of appearance existed among the MMPs detected, we analyzed the urine of 189 patients with prostate or bladder cancer and controls.The approximately 140, greater than 220 kDa, and approximately 190 HMW gelatinase species were identified as MMP-9/tissue inhibitor of metalloproteinase 1 complex, MMP-9 dimer, and ADAMTS-7, respectively. The frequency of detection of any MMP species was significantly higher in urine from prostate and bladder cancer groups than controls. MMP-9 dimer and MMP-9 were independent predictors for distinguishing between patients with prostate and bladder cancer (P less than 0.001 for each) by multivariable analysis.This study is the first to identify a tumor-specific urinary MMP fingerprint that may noninvasively facilitate identification of cancer presence and type. This information may be of diagnostic and prognostic value in the detection and/or clinical monitoring of disease progression and therapeutic efficacy in patients with bladder or prostate cancer. | 18927302
 |
Reduced expression of inducible gelatinase B/matrix metalloproteinase-9 in monocytes from patients with myelodysplastic syndrome: Correlation of inducible levels with the percentage of cytogenetically marked cells and with marrow cellularity.
Mineo Iwata,Manoj Pillai,Aravind Ramakrishnan,Robert C Hackman,H Joachim Deeg,Ghislain Opdenakker,Beverly Torok-Storb,H Joachim Deeg
Blood
109
2007
Show Abstract
Regulatory molecules produced by stromal cells are often membrane bound until cleaved by matrix metalloproteinases (MMPs); cleavage can either activate or inactivate regulatory functions. We report here that marrow stromal cells induce the expression of MMP-9 in monocytes. Induction was contact independent and could be reproduced with recombinant MCP-1/CCL2, whereas IL-6, M-CSF, G-CSF, GM-CSF, IL-8/CXCL8, SDF-1/CXCL12, and MGSA/CXCL1 did not have this effect. Stroma-induced levels of MMP-9 in the monocyte population from healthy donors were relatively consistent, whereas induced levels varied significantly (P < .001) in the CD14+ population from 27 patients with myelodysplastic syndrome (MDS). In patients with a clonal chromosomal marker, the level of inducible MMP-9 expression in the monocyte population was inversely correlated with the percentage of marker-positive cells (n = 11, P = .01), suggesting that the ability to induce MMP-9 may be compromised in clonally derived monocytes. The inducible levels of MMP-9 were also inversely correlated with marrow cellularity observed in biopsies from MDS patients (P < .001). We conclude that monocytes can express MMP-9 in response to stromal factors and that this response may be significantly decreased in MDS-derived monocytes. Full Text Article | 16954500
 |
Increased tissue factor, MMP-8, and D-dimer expression in diabetic patients with unstable advanced carotid atherosclerosis.
Krupinski, J; Turu, MM; Font, MA; Ahmed, N; Sullivan, M; Rubio, F; Badimon, L; Slevin, M
Vascular health and risk management
3
405-12
2007
Show Abstract
Advanced atherogenesis is characterized by the presence of markers of enhanced prothrombotic capacity, attenuated fibrinolysis, and by clinical conditions associated with defective coagulation. Diabetes may be associated with enhanced lesion instability and atherosclerotic plaque rupture. Plaques obtained from 206 patients undergoing carotid endarterectomy were divided into diabetic (type 2) and nondiabetic and analyzed by Western blotting and immunohistochemistry to detect tissue factor (TF), metalloproteinases (MMP)-2, -8, -9, and fibrin/fibrinogen related antigens, and in situ zymography to detect MMP activity. Plasma samples were quantified for TF procoagulant activity, C-reactive protein, fibrinogen and D-dimer. Diabetic and symptomatic patients with hypoechogenic plaques had increased plasma TF activity and D-dimer, compared with those with hyperechogenic plaques (p = 0.03, p = 0.007, respectively). Diabetic, symptomatic patients had higher plasma D-dimer levels than asymptomatic patients (p = 0.03). There was a significant correlation between intramural TF levels and D-dimer in diabetic patients with symptomatic disease (p = 0.001, r2 = 0.4). In diabetic patients, plasma fibrinogen levels were higher in patients with hypoechogenic plaques (p = 0.007). Diabetic patients with ulcerated plaques had higher plasma D-dimer and MMP-8 levels than those with fibrous plaques (p = 0.02, p = 0.01, respectively). This data suggests that currently available circulating markers may be clinically useful to select diabetic patients at higher risk of atherothrombosis. Increased procoagulant activity in diabetic patients may be linked to increased mural remodeling. | 17969370
 |
Intraplaque MMP-8 levels are increased in asymptomatic patients with carotid plaque progression on ultrasound.
Marta Miguel Turu, Jerzy Krupinski, Esther Catena, Ana Rosell, Joan Montaner, Francisco Rubio, Jose Alvarez-Sabin, Marc Cairols, Lina Badimon
Atherosclerosis
187
161-9
2005
Show Abstract
Carotid atherosclerotic plaque remodelling and increased risk of symptomatic plaque rupture seem to be partially mediated by matrix metalloproteinases (MMPs). In this study, we have investigated whether different MMPs are related to carotid atherosclerosis or to recent ischaemic brain disease. Eighty-four consecutive patients undergoing carotid endarterectomy for symptomatic and asymptomatic disease were studied. Plaques were analysed by ultrasound and later by morphology. Plasma MMP-2, MMP-8 and MMP-9 levels were quantified by ELISA. MMP expression and activity in carotid plaques was analysed by Western blotting and in situ zymography. Results were analysed with respect to plaque stability, morphology, symptomatic disease, presence of vascular risk factors and plasma markers of acute inflammation as high sensitivity C-reactive protein (hsCRP), fibrinogen, D-dimer and white blood cell counts. Patients with hypoechogenic plaques on ultrasound had more plasma MMP-8 (p = 0.04) and increased MMP activity as assessed by in situ zymography. Asymptomatic patients with plaque progression had more active intraplaque MMP-8 than asymptomatic patients without plaque progression. Presence of recent intraplaque haemorrhage or past history of CAD was related to increased activity of MMPs as assessed by in situ zymography (p 0.01, CI 95% 0.8-1.0). Plasma MMP-8 and MMP-9, but not MMP-2 levels, decrease with time after ischaemic stroke. Patients with hypertension had more intraplaque active MMP-9 than normotensive (p = 0.03, CI 95% 0.7-1.0). Hypoechogenic carotid plaques had increased MMP activity and asymptomatic patients with plaque progression show increase intraplaque MMP-8 levels. | 16259988
 |
Matrix metalloproteinase expression in basal cell carcinoma: relationship between enzyme profile and collagen fragmentation pattern.
Taskin Yucel, Amar Mutnal, Kevin Fay, Suzanne E G Fligiel, Timothy Wang, Timothy Johnson, Shan R Baker, James Varani
Experimental and molecular pathology
79
151-60
2004
Show Abstract
Matrix metalloproteinases (MMPs) with collagenolytic and gelatinolytic activities are up-regulated in basal cell carcinoma. In the present study we demonstrate that the major collagenolytic enzyme detected is MMP-1 (interstitial collagenase) while gelatinolytic enzymes include both MMP-2 (72-kDa gelatinase A) and MMP-9 (92-kDa gelatinase B). Significant fractions of all three enzymes are present as active forms. In spite of the fact that high levels of gelatinolytic enzymes are present, the major fragmentation products resulting from digestion of intact type I collagen are the 1/4 and 3/4 fragments (products of MMP-1-mediated digestion). Thus, it appears that the gelatinolytic enzymes are not capable of degrading the collagen fragments as rapidly as they are produced. Since previous studies have demonstrated that interaction of interstitial fibroblasts with high molecular weight fragments of type I collagen leads to increased MMP production, the present results suggest a mechanism underlying altered function of stromal elements in the connective tissue adjacent to the growing neoplasm. | 16004981
 |