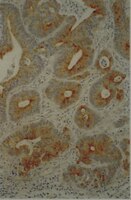
DSG3 facilitates cancer cell growth and invasion through the DSG3-plakoglobin-TCF/LEF-Myc/cyclin D1/MMP signaling pathway.
Chen, YJ; Lee, LY; Chao, YK; Chang, JT; Lu, YC; Li, HF; Chiu, CC; Li, YC; Li, YL; Chiou, JF; Cheng, AJ
PloS one
8
e64088
2013
Show Abstract
Desmoglein 3 (DSG3) is a component of the desmosome, which confers strong cell-cell adhesion. Previously, an oncogenic function of DSG3 has been found in head neck cancer (HNC). Here, we investigated how this molecule contributes to the malignant phenotype. Because DSG3 is associated with plakoglobin, we examined whether these phenotypic alterations were mediated through the plakoglobin molecule. Immunoprecipitation and immunofluorescence staining revealed that DSG3 silencing disrupted its interaction with plakoglobin and induced plakoglobin translocation from the cytoplasm to the nucleus. Knockdown of DSG3 significantly increased the interaction of plakoglobin with the transcriptional factor TCF and suppressed the TCF/LEF transcriptional activity. These effects further conferred to reduced expression of the TCF/LEF downstream target genes, including c-myc, cyclin D1, and MMP-7. Functional analyses showed that DSG3 silencing reduced cell growth and arrested cells at G0/G1 phase. Besides, cell migration and invasion abilities were also decreased. These cellular results were confirmed using tumor xenografts in mice, as DSG3 silencing led to the suppressed tumor growth, plakoglobin translocation and reduced expression of TCF/LEF target genes in tumors. Therefore, our study shows that the desmosomal protein DSG3 additionally functions to regulate malignant phenotypes via nuclear signaling. In conclusion, we found that DSG3 functions as an oncogene and facilitates cancer growth and invasion in HNC cells through the DSG3-plakoglobin-TCF/LEF pathway. | | 23737966
 |
WNT7A regulates tumor growth and progression in ovarian cancer through the WNT/β-catenin pathway.
Yoshioka, S; King, ML; Ran, S; Okuda, H; MacLean, JA; McAsey, ME; Sugino, N; Brard, L; Watabe, K; Hayashi, K
Molecular cancer research : MCR
10
469-82
2011
Show Abstract
Abnormal activation the WNT/β-catenin signaling pathway has been associated with ovarian carcinomas, but a specific WNT ligand and pertinent downstream mechanisms are not fully understood. In this study, we found abundant WNT7A in the epithelium of serous ovarian carcinomas, but not detected in borderline and benign tumors, normal ovary, or endometrioid carcinomas. To characterize the role of WNT7A in ovarian tumor growth and progression, nude mice were injected either intraperitoneally or subcutaneously with WNT7A knocked down SKOV3.ip1 and overexpressed SKOV3 cells. In the intraperitoneal group, mice receiving SKOV3.ip1 cells with reduced WNT7A expression developed significantly fewer tumor lesions. Gross and histologic examination revealed greatly reduced invasion of WNT7A knockdown cells into intestinal mesentery and serosa compared with the control cells. Tumor growth was regulated by loss or overexpression of WNT7A in mice receiving subcutaneous injection as well. In vitro analysis of cell function revealed that cell proliferation, adhesion, and invasion were regulated by WNT7A. The activity of the T-cell factor/lymphoid enhancer factor (TCF/LEF) reporter was stimulated by overexpression of WNT7A in ovarian cancer cells. Cotransfection with WNT7A and FZD5 receptor further increased activity, and this effect was inhibited by cotransfection with SFRP2 or dominant negative TCF4. Overexpression of WNT7A stimulated matrix metalloproteinase 7 (MMP7) promoter, and mutation of TCF-binding sites in MMP7 promoter confirmed that activation of MMP7 promoter by WNT7A was mediated by β-catenin/TCF signaling. Collectively, these results suggest that reexpression of WNT7A during malignant transformation of ovarian epithelial cells plays a critical role in ovarian cancer progression mediated by WNT/β-catenin signaling pathway. | Western Blotting | 22232518
 |
Matrix metalloproteinases in the restorative proctocolectomy pouch of pediatric ulcerative colitis.
Mäkitalo, L; Piekkala, M; Ashorn, M; Pakarinen, M; Koivusalo, A; Karikoski, R; Natunen, J; Saarialho-Kere, U; Rintala, R; Kolho, KL
World journal of gastroenterology
18
4028-36
2011
Show Abstract
To investigate matrix metalloproteinases (MMPs) and their tissue inhibitors (TIMPs) in pouch mucosa of pediatric onset ulcerative colitis (UC).In this cross-sectional study, 28 patients with pediatric onset UC underwent ileal pouch biopsy 13 years (median) after proctocolectomy. Expression of MMPs-3, -7, -8, -9, -12 and -26 and TIMPs-1, -2 and -3 in samples was examined using immunohistochemichal methods, and another biopsy was used to evaluate the grade of histological inflammation. Two investigators independently graded the immunohistochemical specimens in a semiquantitative fashion, using a scale marking staining intensity as follows: 0 = less than 20 positive cells; 1 = 20-50 positive cells; 2 = 50-200 positive cells; 3 = over 20 positive cells. Fecal calprotectin and blood inflammatory markers [serum C-reactive protein (CRP) and erythrocyte sedimentation rate] were determined during a follow-up visit to examine correlations between these markers and the expression of MMPs and TIMPs.Of the 28 patients with pediatric onset UC, nine had not experienced pouchitis, whereas thirteen reported a single episode, and six had recurrent pouchitis (≥ 4 episodes). At the time of the study, six patients required metronidazole. In all of the others, the most recent episode of pouchitis had occurred over one month earlier, and none were on antibiotics. Only four samples depicted no sign of inflammation, and these were all from patients who had not had pouchitis. Two samples were too small to determine the grade of inflammation, but both had suffered pouchitis, the other recurrent. No sample depicted signs of colonic metaplasia. Most pouch samples showed expression of epithelial (e) and stromal (s) MMP-3 (e, n = 22; s, n = 20), MMP-7 (e, n = 28; s, n = 27), MMP-12 (e, n = 20; s, n =24), TIMP-2 (e, n = 23; s, n = 23) and MMP-3 (e, n = 23; s, n = 28) but MMP-8 (e, n = 0; s, n = 1), MMP-9 (e, n = 0; s, n = 9) and MMP-26 (e, n = 0; s, n = 3) and TIMP-1 (n = 0, both) were lacking. In samples with low grade of inflammatory activity, the epithelial MMP-3 and MMP-7 expression was increased (r = -0.614 and r = -0.472, respectively, P less than 0.05 in both). MMPs and TIMPs did not correlate with the markers of inflammation, fecal calprotectin, erythrocyte sedimentation rate, or CRP, with the exception of patients with low fecal calprotectin (less than 100 μg/g) in whom a higher expression of epithelial MMP-7 was found no differences in MMP- or TIMP-profiles were seen in patients with a history of pouchitis compared to ones with no such episodes. Anastomosis with either straight ileoanal anastomosis or ileoanal anastomosis with J-pouch did depict differences in MMP- or TIMP-expression.The expression of MMPs pediatric UC pouch in the long-term shares characteristics with inflammatory bowel disease, but inflammation cannot be classified as a reactivation of the disease. | | 22912554
 |
Prognostic value of E-cadherin, beta-catenin, MMPs (7 and 9), and TIMPs (1 and 2) in patients with colorectal carcinoma.
Fernanda Roca, Laura V Mauro, Ana Morandi, Fernando Bonadeo, Carlos Vaccaro, Guillermo Ojea Quintana, Sergio Specterman, Elisa Bal de Kier Joffé, María Guadalupe Pallotta, Lydia Inés Puricelli, José Lastiri
Journal of surgical oncology
93
151-60
2005
Show Abstract
BACKGROUND AND OBJECTIVES: Therapy of colorectal tumors (CRC) based on histology and clinical factors is insufficient to predict the evolution of each patient. The finding of molecular abnormalities able to differentiate subgroups of patients with bad prognosis will improve our ability to treat them successfully. Our purpose was to analyze retrospectively the prognostic input of E-cadherin, beta-catenin, metalloproteinases (MMPs) (7 and 9), and tissue inhibitors of metalloproteinases (TIMPs) (1 and 2) in patients with a follow-up period of 5 years. METHODS: Antigen expression was analyzed by immunohistochemistry. Prognostic evaluation was performed with the multivariate proportional hazards model. RESULTS: We demonstrated a concomitant loss of E-cadherin and beta-catenin at membranous level and an abnormal accumulation of nuclear beta-catenin. Besides, we found that all MMPs and TIMPs studied were overexpressed in CRC tissue. There was no association between the expression of any of these molecules and the known clinical-pathological parameters employed in CRC pathology. A multivariate analysis demonstrated that the overall survival could be independently predicted by the loss of E-cadherin and the overexpression of TIMP-2. CONCLUSIONS: The expression of E-cadherin and TIMP-2 could be relevant in determining the prognosis of CRC patients and providing a more accurate mechanism for their classification. | | 16425303
 |
Profiling markers of prognosis in colorectal cancer.
Lyall, MS; Dundas, SR; Curran, S; Murray, GI
Clinical cancer research : an official journal of the American Association for Cancer Research
12
1184-91
2005
Show Abstract
Colorectal cancer is one of the most common forms of cancer in developed nations and the incidence of this disease is increasing. There is a need to further stratify prognostically distinct groups of colorectal cancer, and the purpose of this study was to identify prognostically significant immunohistochemical marker profiles in colorectal cancer.In this study, a range (n = 23) of markers [pRb, p16, p21, p27, p53, proliferating cell nuclear antigen, cyclin D1, bcl-2, epidermal growth factor receptor, C-erb-B2, topoisomerase-I, liver fatty acid-binding protein, matrix metalloproteinases (MMP) 1-3, 7, 9, and 13, MT1-MMP, MT2-MMP, and tissue inhibitors of MMP 1-3] of putative prognostic significance have been investigated by immunohistochemistry on formalin-fixed, wax-embedded sections in a series (n = 90) of stage III (Dukes C) colorectal cancers. An immunohistochemical score based on the intensity of immunoreactivity and, where relevant, the proportion of immunoreactive cells was established for each marker.Unsupervised two-dimensional hierarchical cluster analysis identified three distinct cluster groups (designated groups 1-3) with different marker profiles. There were significant survival differences between groups 1 and 2 (log rank = 11.48; P = 0.0007) and between groups 1 and 3 (log rank = 8.32; P = 0.0039). Multivariate analysis showed that the complete marker profile was independently the most significant prognostic factor (hazard ratio, 2.27; 95% confidence interval, 1.15-4.48; P = 0.004).This study has identified an immunohistochemical marker profile of colorectal cancer and showed that it is an independent indicator of prognosis in this type of cancer. | | 16489072
 |
Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in HTLV-I-associated myelopathy.
Umehara, F, et al.
J. Neuropathol. Exp. Neurol., 57: 839-49 (1998)
1998
Show Abstract
Matrix metalloproteinases (MMPs) have been reported to be involved in inflammatory disorders of the central nervous system (CNS). However, little is known about the role of MMPs in the pathogenesis of HTLV-I-associated myelopathy (HAM)/Tropical spastic paraparesis (TSP). To address this issue, we examined the tissue expression and localization of MMPs and their inhibitors, tissue inhibitors of metalloproteinases (TIMPs) in the spinal cord lesions of HAM/TSP using immunohistochemistry. In addition, the blood and cerebrospinal fluid (CSF) levels of MMPs and TIMPs of the patients with HAM/TSP were determined using sandwich enzyme immunoassays (SIA) and gelatin zymography. Immunohistochemical studies revealed that collagen IV and decorin immunoreactivity on the basement membrane of CNS parenchymal vessels was partially disrupted where inflammatory mononuclear cells infiltrated in active-chronic lesions of HAM/TSP. In these lesions, MMP-2 (gelatinase A) was immunostained mainly on the surface of foamy macrophages and lymphocytes, whereas MMP-9 (gelatinase B) expression was positive in the intravascular and perivascular mononuclear cells but not on foamy macrophages. In contrast, inactive chronic lesions of the spinal cords of the HAM/TSP contained fewer MMP-2-positive or MMP-9-positive mononuclear cells than active-chronic lesions. Many parenchymal vessels had thickened vascular walls which showed increased immunoreactivity to decorin. SIA revealed that production levels of MMP-2 and MMP-9 in both blood and CSF were higher in the patients with HAM/TSP than those in non-inflammatory other neurological disease controls (ONDs). Using zymography, proMMP-9 was detected more frequently in the CSF of patients with HAM/TSP than those in ONDs. Taken together, our data indicate that MMP-2 and MMP-9 may play an important role in the blood-brain barrier breakdown and tissue remodeling in the CNS of HAM/TSP. | | 9737547
 |