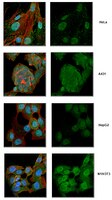
The Ebola virus VP35 protein inhibits activation of interferon regulatory factor 3.
Basler, Christopher F, et al.
J. Virol., 77: 7945-56 (2003)
2003
Show Abstract
The Ebola virus VP35 protein was previously found to act as an interferon (IFN) antagonist which could complement growth of influenza delNS1 virus, a mutant influenza virus lacking the influenza virus IFN antagonist protein, NS1. The Ebola virus VP35 could also prevent the virus- or double-stranded RNA-mediated transcriptional activation of both the beta IFN (IFN-beta) promoter and the IFN-stimulated ISG54 promoter (C. Basler et al., Proc. Natl. Acad. Sci. USA 97:12289-12294, 2000). We now show that VP35 inhibits virus infection-induced transcriptional activation of IFN regulatory factor 3 (IRF-3)-responsive mammalian promoters and that VP35 does not block signaling from the IFN-alpha/beta receptor. The ability of VP35 to inhibit this virus-induced transcription correlates with its ability to block activation of IRF-3, a cellular transcription factor of central importance in initiating the host cell IFN response. We demonstrate that VP35 blocks the Sendai virus-induced activation of two promoters which can be directly activated by IRF-3, namely, the ISG54 promoter and the ISG56 promoter. Further, expression of VP35 prevents the IRF-3-dependent activation of the IFN-alpha4 promoter in response to viral infection. The inhibition of IRF-3 appears to occur through an inhibition of IRF-3 phosphorylation. VP35 blocks virus-induced IRF-3 phosphorylation and subsequent IRF-3 dimerization and nuclear translocation. Consistent with these observations, Ebola virus infection of Vero cells activated neither transcription from the ISG54 promoter nor nuclear accumulation of IRF-3. These data suggest that in Ebola virus-infected cells, VP35 inhibits the induction of antiviral genes, including the IFN-beta gene, by blocking IRF-3 activation. | 12829834
 |
Interferon regulatory factor-3 is an in vivo target of DNA-PK.
Karpova, Alla Y, et al.
Proc. Natl. Acad. Sci. U.S.A., 99: 2818-23 (2002)
2002
Show Abstract
Eukaryotic cells have evolved complex signaling networks to sense environmental stress and to repair stress-induced damage. IFN regulatory factor-3 (IRF-3) is a transcription factor that plays a central role in the host response to viral infection. Although the main activity of IRF-3 characterized to date has been its role in the induction of IFN-alpha and -beta after virus infection, recent evidence indicates additional roles for IRF-3 in the response to DNA damage and in virus-induced apoptosis. Here we identify IRF-3 as the first in vivo target for DNA-dependent protein kinase (DNA-PK). Phosphorylation of IRF-3 by DNA-PK after virus infection results in its nuclear retention and delayed proteolysis. These results expand the known roles of DNA-PK and provide a functional link between the cellular machineries that regulate the innate immune response and that sense and respond to DNA damage. As such this study contributes to a more integrated view of the cellular responses to various cellular stress signals. | 11867762
 |
Activation of interferon regulatory factor 3 is inhibited by the influenza A virus NS1 protein.
Talon, J, et al.
J. Virol., 74: 7989-96 (2000)
1999
Show Abstract
We present a novel mechanism by which viruses may inhibit the alpha/beta interferon (IFN-alpha/beta) cascade. The double-stranded RNA (dsRNA) binding protein NS1 of influenza virus is shown to prevent the potent antiviral interferon response by inhibiting the activation of interferon regulatory factor 3 (IRF-3), a key regulator of IFN-alpha/beta gene expression. IRF-3 activation and, as a consequence, IFN-beta mRNA induction are inhibited in wild-type (PR8) influenza virus-infected cells but not in cells infected with an isogenic virus lacking the NS1 gene (delNS1 virus). Furthermore, NS1 is shown to be a general inhibitor of the interferon signaling pathway. Inhibition of IRF-3 activation can be achieved by the expression of wild-type NS1 in trans, not only in delNS1 virus-infected cells but also in cells infected with a heterologous RNA virus (Newcastle disease virus). We propose that inhibition of IRF-3 activation by a dsRNA binding protein significantly contributes to the virulence of influenza A viruses and possibly to that of other viruses. | 10933707
 |
Human papillomavirus 16 E6 oncoprotein binds to interferon regulatory factor-3 and inhibits its transcriptional activity.
Ronco, L V, et al.
Genes Dev., 12: 2061-72 (1998)
1998
Show Abstract
Interferon regulatory factor-3 (IRF-3) was found to specifically interact with HPV16 E6 in a yeast two-hybrid screen. IRF-3 is activated by the presence of double-stranded RNA or by virus infection to form a stable complex with other transcriptional regulators that bind to the regulatory elements of the IFNbeta promoter. We show that IRF-3 is a potent transcriptional activator and demonstrate that HPV16 E6 can inhibit its transactivation function. The expression of HPV16 E6 in primary human keratinocytes inhibits the induction of IFNbeta mRNA following Sendai virus infection. The binding of HPV16 E6 to IRF-3 does not result in its ubiquitination or degradation. We propose that the interaction of E6 with IRF-3 and the inhibition of IRF-3's transcriptional activity may provide the virus a means to circumvent the normal antiviral response of an HPV16-infected cell. | 9649509
 |