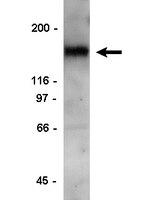
Syndecan-1 couples the insulin-like growth factor-1 receptor to inside-out integrin activation.
Beauvais, DM; Rapraeger, AC
Journal of cell science
123
3796-807
2009
Show Abstract
Syndecan-1 (Sdc1) engages and activates the αvβ3 (and/or αvβ5) integrin when clustered in human carcinoma and endothelial cells. Although the engagement is extracellular, the activation mechanism is cytoplasmic. This talin-dependent, inside-out signaling pathway is activated downstream of the insulin-like growth factor-1 receptor (IGF1R), whose kinase activity is triggered by Sdc1 clustering. In vitro binding assays using purified receptors suggest that association of the Sdc1 ectodomain with the integrin provides a 'docking face' for IGF1R. IGF1R docking and activation of the associated integrin is blocked by synstatin (SSTN(92-119)), a peptide derived from the integrin engagement site in Sdc1. IGF1R colocalizes with αvβ3 integrin and Sdc1 in focal contacts, but fails to associate with or activate the integrin in cells either lacking Sdc1 or expressing Sdc1(Δ67-121), a mutant that is unable to form the Sdc1-integrin-IGF1R ternary complex. Integrin activation is also blocked by IGF1R inhibitors or by silencing IGF1R or talin expression with small-interfering RNAs (siRNAs). In both cases, expression of the constitutively active talin F23 head domain rescues integrin activation. We recently reported that SSTN(92-119) blocks angiogenesis and impairs tumor growth in mice, therefore this Sdc1-mediated integrin regulatory mechanism might be a crucial regulator of disease processes known to rely on these integrins, including tumor cell metastasis and tumor-induced angiogenesis. | | 20971705
 |
Immunogold labeling of insulin growth factor-I receptors in elderly human skeletal muscle.
Maria Urso, Arthur Cosmas, Maria Fiatarone Singh, Thomas Manfredi
Scanning
27
208-12
2004
Show Abstract
Age-associated muscle wasting, or sarcopenia, can be delayed or reversed with interventions, including exercise and pharmaceutical agents. Mapping morphometric changes in the skeletal muscle insulin growth factor 1 receptor can provide valuable information regarding mechanisms controlling muscle protein metabolism. Immunocolloidal gold labeling is a powerful immunocytochemistry procedure for detecting antigens at the ultrastructural level, providing plausible biological markers of cell and tissue adaptations to stimuli. The intent here was to employ immunogold labeling to identify, localize, and quantify the insulin growth factor receptor-I (IGF-IR) in elderly human skeletal muscle. Needle biopsy specimens of the leg vastus lateralis muscle were fixed with 1% glutaraldehyde and 4% paraformaldehyde, dehydrated, and embedded in LR white resin. Pilot experiments were carried out to establish optimal dilutions of primary and secondary antibodies and to employ controls to establish staining specificity. The 6 nm gold particles were first evident when viewed at transmission electron microscopy (TEM) magnifications at 54,000x and clearest at 71,000x. Consistencies were noted in the staining patterns, with the majority of particles lying in proximity to the myofilaments. Gold particles were also found randomly along the outer membrane of the sarcolemma and the mitochondrial membranes. National Institutes of Health (NIH) Image 1.55 version software was used to measure receptor density (NIH, Bethesda, Md., USA). It appears that immunogold labeling of postembedded tissue samples is a sensitive method for detecting IGF-I receptors at the ultrastructural level. | | 16089305
 |
Tyrosine phosphorylation of paxillin and focal adhesion kinase during insulin-like growth factor-I-stimulated lamellipodial advance.
Leventhal, P S, et al.
J. Biol. Chem., 272: 5214-8 (1997)
1997
Show Abstract
In the current studies, we examined whether focal adhesion kinase (FAK) and paxillin play a role in insulin-like growth factor-I (IGF-I)-stimulated morphological changes in neuronal cells. In SH-SY5Y human neuroblastoma cells, 10 nM IGF-I enhanced the extension of lamellipodia within 30 min. Scanning electron microscopy and staining with rhodamine-phalloidin showed that these lamellipodia displayed ruffles, filopodia, and a distinct meshwork of actin filaments. Immunofluorescent staining identified focal concentrations of FAK, paxillin, and phosphotyrosine within the lamellipodia. Immunoprecipitation experiments revealed that FAK and paxillin are tyrosine-phosphorylated during IGF-I-stimulated lamellipodial extension. Maximal phosphorylation of FAK and paxillin was observed 15-30 min after the addition of 10 nM IGF-I, whereas maximal IGF-I receptor phosphorylation occurred within 5 min. FAK, paxillin, and IGF-I receptor tyrosine phosphorylation had similar concentration-response curves and were inhibited by the receptor blocking antibody alphaIR-3. These results indicate that FAK and paxillin are tyrosine-phosphorylated during IGF-I-stimulated lamellipodial advance and suggest that the tyrosine phosphorylation of these two proteins helps mediate IGF-I-stimulated cell and growth cone motility. These responses contrast directly with recent reports showing insulin-stimulated dephosphorylation of FAK and paxillin. | Immunocytochemistry | 9030591
 |
Regulation of insulin-like growth factor I receptors in diabetic mesangial cells.
Oemar, B S, et al.
J. Biol. Chem., 266: 2369-73 (1991)
1991
Show Abstract
Mesangial cells are thought to play a central role in the renal complications of diabetes mellitus. Insulin-like growth factor I (IGF-I) has been found to promote mesangial cell proliferation and regulate normal mesangial cell function in an autocrine and/or paracrine fashion. To gain further insight into the potential regulatory role IGF-I may play in mesangial cell function in diabetes, IGF-I receptors were analyzed in mesangial cells isolated from diabetic mice (db/db) and their control littermates (db/m). Mesangial cells isolated from db/db mice exhibited higher levels of IGF-I receptors compared to cells from db/m mice. Insulin receptors were not detectable in either cell type by binding analyses; however, immunoblot analysis revealed insulin receptor alpha-subunits in wheat germ agglutinin-Sepharose-purified membranes from db/db cells. Northern blot analysis further indicated a lack of detectable insulin receptor mRNA in db/m cells, whereas db/db cells expressed multiple insulin receptor mRNA transcripts. Both IGF-I and insulin receptor mRNA levels were increased in db/db cells grown in the presence of high glucose (28 mM), whereas the receptor protein levels remained relatively constant or increased, respectively. This increased expression of IGF-I and insulin receptors in diabetic mesangial cells may have an important role in the development of diabetic nephropathy. | | 1846626
 |
Identification of retinal insulin receptors using site-specific antibodies to a carboxyl-terminal peptide of the human insulin receptor alpha-subunit. Up-regulation of neuronal insulin receptors in diabetes.
Rosenzweig, S A, et al.
J. Biol. Chem., 265: 18030-4 (1990)
1990
Show Abstract
Insulin receptor-specific polyclonal antipeptide serum was generated against a synthetic pentadecapeptide (residues 657-670) of the deduced amino acid sequence of human insulin proreceptor cDNA for use in the analysis of insulin receptors in the retina. The affinity-purified antibodies recognized peptide antigen but not keyhole limpet hemocyanin as determined by dot blot analysis and solid phase radioimmunoassay. Addition of either synthetic peptide or the affinity-purified serum had no effect on 125I-insulin binding to placental membranes or to cells in culture. alpha-Subunits of approximately 125 kDa from human placental membranes and liver membranes were labeled by immunoblot analysis with this antiserum. In membranes isolated from human retina and brain, two classes of alpha-subunits of approximately 125 and 115 kDa were detectable. The 115-kDa subunit was neuraminidase resistant whereas the 125-kDa subunit was digested to a band of 115 kDa, indicating that these bands represent peripheral and neuronal receptors, respectively. Analysis of human retinas obtained from type I diabetic donors revealed an increased level of neuronal receptor as compared with normal retinas. These data indicate that human retina expresses neuronal insulin receptor subtypes that are up-regulated in diabetes. | | 2211678
 |