Astrocyte plasticity revealed by adaptations to severe proteotoxic stress.
Titler, Amanda M, et al.
Cell Tissue Res., 352: 427-43 (2013)
2013
Show Abstract
Neurodegeneration is characterized by an accumulation of misfolded proteins in neurons. It is less well appreciated that glia often also accumulate misfolded proteins. However, glia are highly plastic and may adapt to stress readily. Endogenous adaptations to stress can be measured by challenging stressed cells with a second hit and then measuring viability. For example, subtoxic stress can elicit preconditioning or tolerance against second hits. However, it is not known if severe stress that kills half the population can elicit endogenous adaptations in the remaining survivors. Glia, with their resilient nature, offer an ideal model in which to test this new hypothesis. The present study is the first demonstration that astrocytes surviving one LC50 hit of the proteasome inhibitor MG132 were protected against a second MG132 hit. ATP loss in response to the second hit was also prevented. MG132 caused compensatory rises in stress-sensitive heat shock proteins. However, stressed astrocytes exhibited an even greater rise in ubiquitin-conjugated proteins upon the second hit, illustrating the severity of the proteotoxicity and verifying the continued impact of MG132. Despite this stress, MG132-pretreated astrocytes were completely prevented from losing glutathione with the second hit. Furthermore, inhibiting glutathione synthesis rendered astrocytes sensitive to the second hit, unmasking the cumulative impact of two hits by removal of an endogenous adaptation. These findings suggest that stressed astrocytes become progressively harder to kill by virtue of antioxidant defenses. Such plasticity may permit astrocytes under severe stress to better support neurons and help explain the protracted nature of neurodegeneration. | | 23420451
 |
Neocortex and allocortex respond differentially to cellular stress in vitro and aging in vivo.
Posimo, JM; Titler, AM; Choi, HJ; Unnithan, AS; Leak, RK
PloS one
8
e58596
2013
Show Abstract
In Parkinson's and Alzheimer's diseases, the allocortex accumulates aggregated proteins such as synuclein and tau well before neocortex. We present a new high-throughput model of this topographic difference by microdissecting neocortex and allocortex from the postnatal rat and treating them in parallel fashion with toxins. Allocortical cultures were more vulnerable to low concentrations of the proteasome inhibitors MG132 and PSI but not the oxidative poison H2O2. The proteasome appeared to be more impaired in allocortex because MG132 raised ubiquitin-conjugated proteins and lowered proteasome activity in allocortex more than neocortex. Allocortex cultures were more vulnerable to MG132 despite greater MG132-induced rises in heat shock protein 70, heme oxygenase 1, and catalase. Proteasome subunits PA700 and PA28 were also higher in allocortex cultures, suggesting compensatory adaptations to greater proteasome impairment. Glutathione and ceruloplasmin were not robustly MG132-responsive and were basally higher in neocortical cultures. Notably, neocortex cultures became as vulnerable to MG132 as allocortex when glutathione synthesis or autophagic defenses were inhibited. Conversely, the glutathione precursor N-acetyl cysteine rendered allocortex resilient to MG132. Glutathione and ceruloplasmin levels were then examined in vivo as a function of age because aging is a natural model of proteasome inhibition and oxidative stress. Allocortical glutathione levels rose linearly with age but were similar to neocortex in whole tissue lysates. In contrast, ceruloplasmin levels were strikingly higher in neocortex at all ages and rose linearly until middle age. PA28 levels rose with age and were higher in allocortex in vivo, also paralleling in vitro data. These neo- and allocortical differences have implications for the many studies that treat the telencephalic mantle as a single unit. Our observations suggest that the topographic progression of protein aggregations through the cerebrum may reflect differential responses to low level protein-misfolding stress but also reveal impressive compensatory adaptations in allocortex. | Western Blotting | 23536801
 |
Rescue from a two hit, high-throughput model of neurodegeneration with N-acetyl cysteine.
Ajay S Unnithan,Hailey J H Choi,Amanda M Titler,Jessica M Posimo,Rehana K Leak
Neurochemistry international
61
2011
Show Abstract
Postmortem tissue from patients with neurodegeneration exhibits protein-misfolding stress and reduced proteasome activity. This hallmark burden of proteotoxic stress has led to the term proteinopathies for neurodegenerative diseases. Proteinopathies may also be exacerbated by previous insults, according to the two hit hypothesis of accelerated neurodegeneration. In order to model the response to two successive insults in a high-throughput fashion, we exposed the neuronal cell line N2a to two hits of the proteasome inhibitor MG132 and performed three unbiased viability assays. MG132 toxicity was synergistically exacerbated following sequential hits provided the first hit was high enough to be toxic. This accelerated viability loss was apparent by (1) a nuclear and cytoplasmic stain (DRAQ5+Sapphire), (2) immunocytochemistry for a cytoskeletal marker (α-tubulin), and (3) ATP levels (Cell Titer Glo). Ubiquitin-conjugated proteins were raised by toxic, but not subtoxic MG132, and were thus correlated with toxicity exacerbation at higher doses. We hypothesized that levels of autophagic and antioxidant defenses would be reduced with toxic, but not subtoxic MG132, explaining their differential impact on a second hit. However, proteins involved in chaperone-mediated autophagy were raised by toxic MG132, not reduced. Furthermore, inhibiting autophagy enhanced the toxicity of both subtoxic and toxic MG132 as well as of dual hits, suggesting that autophagic removal of cellular debris protected against proteasome inhibition. Two toxic hits of MG132 synergistically decreased the antioxidant glutathione. The glutathione precursor N-acetyl cysteine reversed this glutathione loss and prevented the toxic response to dual hits by all three assays. Dietary supplementation with N-acetyl cysteine benefits Alzheimer's patients and is currently undergoing clinical trials in Parkinson's disease. The present report is the first demonstration that this versatile compound is protective against synergistic loss of viability as well as of glutathione following unrelenting, sequential hits of proteotoxic stress as may occur in the diseased brain. | | 22691629
 |
Single-cell redox imaging demonstrates a distinctive response of dopaminergic neurons to oxidative insults.
Horowitz, MP; Milanese, C; Di Maio, R; Hu, X; Montero, LM; Sanders, LH; Tapias, V; Sepe, S; van Cappellen, WA; Burton, EA; Greenamyre, JT; Mastroberardino, PG
Antioxidants & redox signaling
15
855-71
2010
Show Abstract
The study of the intracellular oxido-reductive (redox) state is of extreme relevance to the dopamine (DA) neurons of the substantia nigra pars compacta. These cells possess a distinct physiology intrinsically associated with elevated reactive oxygen species production, and they selectively degenerate in Parkinson's disease under oxidative stress conditions. To test the hypothesis that these cells display a unique redox response to mild, physiologically relevant oxidative insults when compared with other neuronal populations, we sought to develop a novel method for quantitatively assessing mild variations in intracellular redox state.We have developed a new imaging strategy to study redox variations in single cells, which is sensitive enough to detect changes within the physiological range. We studied DA neurons' physiological redox response in biological systems of increasing complexity--from primary cultures to zebrafish larvae, to mammalian brains-and identified a redox response that is distinctive for substantia nigra pars compacta DA neurons. We studied simultaneously, and in the same cells, redox state and signaling activation and found that these phenomena are synchronized.The redox histochemistry method we have developed allows for sensitive quantification of intracellular redox state in situ. As this method is compatible with traditional immunohistochemical techniques, it can be applied to diverse settings to investigate, in theory, any cell type of interest.Although the technique we have developed is of general interest, these findings provide insights into the biology of DA neurons in health and disease and may have implications for therapeutic intervention. | | 21395478
 |
Subcellular immunocytochemical analysis detects the highest concentrations of glutathione in mitochondria and not in plastids.
Zechmann, B, et al.
J. Exp. Bot., 59: 4017-27 (2008)
2008
Show Abstract
The tripeptide glutathione is a major antioxidant and redox buffer with multiple roles in plant metabolism. Glutathione biosynthesis is restricted to the cytosol and the plastids and the product is distributed to the various organelles by unknown mechanisms. In the present study immunogold cytochemistry based on anti-glutathione antisera and transmission electron microscopy was used to determine the relative concentration of glutathione in different organelles of Arabidopsis thaliana leaf and root cells. Glutathione-specific labelling was detected in all cellular compartments except the apoplast and the vacuole. The highest glutathione content was surprisingly not found in plastids, which have been described before as a major site of glutathione accumulation, but in mitochondria which lack the capacity for glutathione biosynthesis. Mitochondria of both leaf and root cells contained 7-fold and 4-fold, respectively, higher glutathione levels than plastids while the density of glutathione labelling in the cytosol, nuclei, and peroxisomes was intermediate. The accuracy of the glutathione labelling is supported by two observations. First, pre-adsorption of the anti-glutathione antisera with glutathione reduced the density of the gold particles in all organelles to background levels. Second, the overall glutathione-labelling density was reduced by about 90% in leaves of the glutathione-deficient Arabidopsis mutant pad2-1 and increased in transgenic plants with enhanced glutathione accumulation. Hence, there was a strong correlation between immunocytochemical and biochemical data of glutathione accumulation. Interestingly, the glutathione labelling of mitochondria in pad2-1 remained very similar to wild-type plants thus suggesting that the high mitochondrial glutathione content is maintained in a situation of permanent glutathione-deficiency at the expense of other glutathione pools. High and constant levels of glutathione in mitochondria appear to be particularly important in cell survival strategies and it is predicted that mitochondria must have highly competitive mitochondrial glutathione uptake systems. The present results underline the suggestion that subcellular glutathione concentrations are not controlled by a global mechanism but are controlled on an individual basis and it is therefore not possible to conclude from global biochemical glutathione analysis on the status of the various organellar pools. | | 18977750
 |
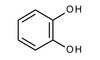
Intracellular adaptations of glutathione content in Cucurbita pepo L. induced by treatment with reduced glutathione and buthionine sulfoximine.
Zechmann, B, et al.
Protoplasma, 227: 197-209 (2006)
2005
Show Abstract
The intracellular effects of GSH (reduced glutathione) and BSO (buthionine sulfoximine) treatment on glutathione content were investigated with immunogold labeling in individual cellular compartments of Cucurbita pepo L. seedlings. Generally, GSH treatment led to increased levels of glutathione in roots and leaves (up to 3.5-fold in nuclei), whereas BSO treatment significantly decreased glutathione content in all organs. Transmission electron microscopy revealed that glutathione levels in mitochondria, which showed the highest glutathione labeling density of all compartments, remained generally unaffected by both treatments. Since glutathione within mitochondria is involved in the regulation of cell death, these results indicate that high and stable levels of glutathione in mitochondria play an important role in cell survival strategies. BSO treatment significantly decreased glutathione levels (1) in roots by about 78% in plastids and 60.8% in the cytosol and (2) in cotyledons by about 55% in the cytosol and 38.6% in plastids. After a short recovery period, glutathione levels were significantly increased in plastids and the cytosol of root tip cells (up to 3.7-fold) and back to control values in cotyledons. These results indicate that plastids, either alone or together with the cytosol, are the main center of glutathione synthesis in leaves as well as in roots. After GSH treatment for 24 h, severe ultrastructural damage related to increased levels of glutathione was found in roots, in all organelles except mitochondria. Possible negative effects of GSH treatment leading to the observed ultrastructural damage are discussed. | | 16520878
 |
Changes in the subcellular distribution of glutathione during virus infection in Cucurbita pepo (L.).
Zechmann, B, et al.
Plant Biol (Stuttg), 7: 49-57 (2005)
2004
Show Abstract
Changes in the subcellular distribution and quantification of glutathione were studied with electron microscopic immunogold cytochemistry in Zucchini yellow mosaic virus (ZYMV)-infected Styrian pumpkin plants (Cucurbita pepo L. ssp. pepo var. styriaca Greb.) two weeks after inoculation. The amount of gold particles bound to glutathione was statistically evaluated for different cell structures, including mitochondria, plastids, nuclei, peroxisomes, and cytosol. In general, ZYMV-infected plants showed higher gold labelling density in intact mesophyll cells of the 5th (older leaves) and the youngest fully developed leaves (younger leaves), and decreased levels of glutathione within root tip cells when compared to the control. In general, within older and younger leaves the highest amount of gold particles was found in mitochondria and the lowest amount in plastids. In ZYMV-infected older leaves, an increase in glutathione was found in peroxisomes (1.7-fold), the cytosol (1.6-fold), mitochondria (1.4-fold), and nuclei (1.2-fold), whereas glutathione levels in plastids did not differ significantly when compared to control cells. In ZYMV-infected younger leaves elevated glutathione contents were found in the cytosol (3-fold), nuclei (2.1-fold), peroxisomes (1.8-fold), and plastids (1.5-fold), whereas mitochondria showed an insignificant decrease in glutathione levels in comparison to the control. In root tip cells of ZYMV-infected plants the amount of gold particles bound to glutathione was decreased in all investigated cell structures by between 0.7- to 0.8-fold. Additionally, total glutathione contents were determined in older and younger leaves using high-performance liquid chromatography (HPLC), which revealed no significant differences between control and ZYMV-infected leaves. The relevance of the results of both methods were compared and are discussed. | | 15666214
 |
Temperature effects on the MgATP-induced electron transfer between the nitrogenase proteins from Azotobacter vinelandii.
Mensink, R E and Haaker, H
Eur. J. Biochem., 208: 295-9 (1992)
1992
Show Abstract
The temperature dependence of the pre-steady-state MgATP-dependent electron transfer from the MoFe protein to the Fe protein of the nitrogenase from Azotobacter vinelandii has been investigated between 6 degrees C and 31 degrees C by stopped-flow spectrophotometry. Below 14 degrees C, the data are consistent with a model in which interaction of MgATP with nitrogenase is fast and irreversible, and is followed by reversible electron transfer. From the extent and from the rate of the absorbance change, the rate constants for electron transfer from Fe protein to MoFe protein and of the reverse reaction were calculated. The direct rate constant increases with temperature (6-14 degrees C) from about 1 s-1 to about 26 s-1. The rate constant for the reverse reaction was found to be approximately 4 s-1 and invariant with the reaction temperature. Analysis of the data obtained in the temperature range between 6 degrees C and 12 degrees C within the framework of the transition-state theory show that electron transfer from the Fe protein to the MoFe protein occurs via a highly disordered transition state with activation parameters delta H(0) ++ = 289 kJ.mol-1 and delta S(0) ++ = 792 J.K-1.mol-1. The Eyring plot of the stopped-flow data displays an inflection point around 14 degrees C. From the stopped-flow data obtained between 18 degrees and 27 degrees C the activation parameters delta H(0) ++ and delta S(0) ++ for the reduction of the MoFe protein by Fe protein are calculated to be 90 kJ.mol-1 and 99 J.K-1.mol-1 respectively. A second inflection point in the Eyring plot could exist around 28 degrees C. | | 1521527
 |