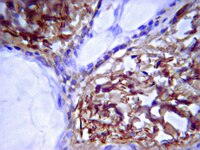
Mizoribine suppresses the progression of experimental peritoneal fibrosis in a rat model.
Shunsuke Takahashi, Yoshihiko Taniguchi, Ayumu Nakashima, Tetsuji Arakawa, Toru Kawai, Shigehiro Doi, Takafumi Ito, Takao Masaki, Nobuoki Kohno, Noriaki Yorioka, Shunsuke Takahashi, Yoshihiko Taniguchi, Ayumu Nakashima, Tetsuji Arakawa, Toru Kawai, Shigehiro Doi, Takafumi Ito, Takao Masaki, Nobuoki Kohno, Noriaki Yorioka
Nephron. Experimental nephrology
112
e59-69
2009
Show Abstract
BACKGROUND/AIMS: Peritoneal fibrosis is a serious complication of peritoneal dialysis (PD). It has been reported that administration of mizoribine, an effective immunosuppressant, ameliorated renal fibrosis in a rat model of unilateral ureteral obstruction. We therefore examined the effects of mizoribine in an experimental model of peritoneal fibrosis. METHODS: 24 rats were given a daily intraperitoneal injection of chlorhexidine gluconate and ethanol dissolved in saline. The rats were divided into three groups (n = 8 per group) that received either vehicle or mizoribine at a dose of 2 or 8 mg/kg once a day. 28 days after the start of the treatments the rats were sacrificed and peritoneal tissue samples collected. Macrophage infiltration (ED1), myofibroblast accumulation (alpha-smooth muscle actin (SMA)) and expression of type III collagen, transforming growth factor (TGF)-beta and monocyte chemotactic protein-1 (MCP-1) were examined by immunohistochemistry. RESULTS: Mizoribine significantly suppressed submesothelial zone thickening and reduced macrophage infiltration. Mizoribine also reduced collagen III(+) area and decreased the number of alpha-SMA(+), TGF-beta(+) and MCP-1(+) cells. The magnitude of the changes observed was dose-dependent. CONCLUSION: The administration of mizoribine prevented the progression of peritoneal fibrosis in this rat model. Mizoribine may represent a novel therapy for peritoneal sclerosis in patients undergoing long-term PD. | 19390220
 |
Formation of smooth muscle alpha actin filaments in CD34+ bone marrow cells on arterial elastic laminae: potential role of SH2 domain-containing protein tyrosine phosphatase-1.
Shu Q Liu, Brandon J Tefft, Andy Zhang, Li-Qun Zhang, Yu H Wu
Matrix biology : journal of the International Society for Matrix Biology
27
282-94
2008
Show Abstract
Arterial smooth muscle cells (SMCs) are present in the elastic lamina-containing media, suggesting that the elastic laminae may regulate the development of SMCs. Here, we investigated the role of elastic laminae in regulating the formation of SM alpha actin filaments in mouse CD34+ bone marrow cells and the role of a protein tyrosine phosphatase, SH2 domain-containing protein tyrosine phosphatase (SHP)-1, in the mediation of this process. Mouse CD34+ bone marrow cells were isolated by magnetic separation and used for assessing the influence of elastic laminae and collagen matrix on the formation of SM alpha actin filaments. CD34+ cells with transgenic SHP-1 knockout or siRNA-mediated SHP-1 knockdown were used to assess the role of SHP-1 in mediating the formation of SM alpha actin filaments. In cell culture tests, elastic laminae, but not collagen matrix, stimulated the formation of SM alpha actin filaments in CD34+ cells. The phosphatase SHP-1 mediated the stimulatory effect of elastic laminae. The interaction of CD34+ cells with elastic laminae, but not with collagen matrix, induced activation of SHP-1. The suppression of SHP-1 by transgenic SHP-1 knockout or siRNA-mediated SHP-1 knockdown significantly reduced the formation of SM alpha actin filaments in CD34+ cells cultured on elastic laminae. The in vitro observations were confirmed by using an in vivo model of implantation of elastic lamina and collagen matrix scaffolds into the aorta. These observations suggest that elastic laminae stimulate the formation of SM alpha actin filaments in CD34+ bone marrow cells and SHP-1 mediates the stimulatory effect of elastic laminae. | 18258420
 |
MR three-dimensional molecular imaging of intramural biomarkers with targeted nanoparticles.
Tillmann Cyrus, Dana R Abendschein, Shelton D Caruthers, Thomas D Harris, Veronica Glattauer, Jerome A Werkmeister, John A M Ramshaw, Samuel A Wickline, Gregory M Lanza
Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance
8
535-41
2005
Show Abstract
In this study, porcine carotid arteries were subjected to balloon overstretch injury followed by local delivery of paramagnetic nanoparticles targeted to alphavbeta3-integrin expressed by smooth muscle cells or collagen III within the extracellular matrix. Carotid T1-weighted angiography and vascular imaging was performed at 1.5T. While MR angiograms were indistinguishable between control and targeted vessel segments, alphavbeta3-integrin-and collagen Ill-targeted nanoparticles spatially delineated patterns and volumes of stretch injury. In conclusion, MR molecular imaging with alphavbeta3-integrin or collagen Ill-targeted nanoparticles enables the non-invasive, three-dimensional characterization of arterial pathology unanticipated from routine angiography. | 16755843
 |