Using the avian mutant talpid2 as a disease model for understanding the oral-facial phenotypes of oral-facial-digital syndrome.
Schock, EN; Chang, CF; Struve, JN; Chang, YT; Chang, J; Delany, ME; Brugmann, SA
Disease models & mechanisms
8
855-66
2015
Show Abstract
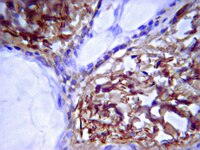
Oral-facial-digital syndrome (OFD) is a ciliopathy that is characterized by oral-facial abnormalities, including cleft lip and/or palate, broad nasal root, dental anomalies, micrognathia and glossal defects. In addition, these individuals have several other characteristic abnormalities that are typical of a ciliopathy, including polysyndactyly, polycystic kidneys and hypoplasia of the cerebellum. Recently, a subset of OFD cases in humans has been linked to mutations in the centriolar protein C2 Ca(2+)-dependent domain-containing 3 (C2CD3). Our previous work identified mutations in C2CD3 as the causal genetic lesion for the avian talpid(2) mutant. Based on this common genetic etiology, we re-examined the talpid(2) mutant biochemically and phenotypically for characteristics of OFD. We found that, as in OFD-affected individuals, protein-protein interactions between C2CD3 and oral-facial-digital syndrome 1 protein (OFD1) are reduced in talpid(2) cells. Furthermore, we found that all common phenotypes were conserved between OFD-affected individuals and avian talpid(2) mutants. In light of these findings, we utilized the talpid(2) model to examine the cellular basis for the oral-facial phenotypes present in OFD. Specifically, we examined the development and differentiation of cranial neural crest cells (CNCCs) when C2CD3-dependent ciliogenesis was impaired. Our studies suggest that although disruptions of C2CD3-dependent ciliogenesis do not affect CNCC specification or proliferation, CNCC migration and differentiation are disrupted. Loss of C2CD3-dependent ciliogenesis affects the dispersion and directional persistence of migratory CNCCs. Furthermore, loss of C2CD3-dependent ciliogenesis results in dysmorphic and enlarged CNCC-derived facial cartilages. Thus, these findings suggest that aberrant CNCC migration and differentiation could contribute to the pathology of oral-facial defects in OFD. | Immunoblotting (Western) | 26044959
 |
Mesenchymal stem cells in rabbit meniscus and bone marrow exhibit a similar feature but a heterogeneous multi-differentiation potential: superiority of meniscus as a cell source for meniscus repair.
Ding, Z; Huang, H
BMC musculoskeletal disorders
16
65
2015
Show Abstract
The restoration of damaged meniscus has always been a challenge due to its limited healing capacity. Recently, bone marrow-derived mesenchymal stem cells (BMSCs) provide a promising alternative to repair meniscal defects. However, BMSCs are not ideal chondroprogenitor cells for meniscus repair because they have a high propensity for cartilage hypertrophy and bone formation. Our hypothesis is that mesenchymal stem cells (MSCs) reside in meniscus maintain specific traits distinct from others which may be more conducive to meniscus regeneration.MSCs were isolated from bone marrow and menisci of the rabbits. The similarities and differences between BMSCs and MMSCs were investigated in vitro by a cell culture model, ex vivo by a rabbit meniscus defect model and in vivo by a nude rat implantation model using histochemistry, immunocytochemistry, qRT-PCR and western blotting.Our data showed that two types of MSCs have universal stem cell characteristics including clonogenicity, multi-potency and self-renewal capacity. They both express stem cell markers including SSEA-4, Nanog, nucleostemin, strol-1, CD44 and CD90. However, MMSCs differed from BMSCs. MMSC colonies were much smaller and grew more slowly than BMSC colonies. Moreover, fewer MMSCs expressed CD34 than BMSCs. Finally, MMSCs always appeared a pronounced tendency to chondrogenic differentiation while BMSCs exhibited significantly greater osteogenic potential, whatever in vitro and in vivo.This study shows the similarities and differences between MMSCs and BMSCs for the first time. MMSCs are a promising source of mesenchymal stem cells in repairing meniscus defect. | | 25887689
 |
Low-density lipoprotein receptor-related protein 5 governs Wnt-mediated osteoarthritic cartilage destruction.
Shin, Y; Huh, YH; Kim, K; Kim, S; Park, KH; Koh, JT; Chun, JS; Ryu, JH
Arthritis research & therapy
16
R37
2014
Show Abstract
Wnt ligands bind to low-density lipoprotein receptor-related protein (LRP) 5 or 6, triggering a cascade of downstream events that include β-catenin signaling. Here we explored the roles of LRP5 in interleukin 1β (IL-1β)- or Wnt-mediated osteoarthritic (OA) cartilage destruction in mice.The expression levels of LRP5, type II collagen, and catabolic factors were determined in mouse articular chondrocytes, human OA cartilage, and mouse experimental OA cartilage. Experimental OA in wild-type, Lrp5 total knockout (Lrp5⁻/⁻) and chondrocyte-specific knockout (Lrp5fl/fl;Col2a1-cre) mice was caused by aging, destabilization of the medial meniscus (DMM), or intra-articular injection of collagenase. The role of LRP5 was confirmed in vitro by small interfering RNA-mediated knockdown of Lrp5 or in Lrp5⁻/⁻ cells treated with IL-1β or Wnt proteins.IL-1β treatment increased the expression of LRP5 (but not LRP6) via JNK and NF-κB signaling. LRP5 was upregulated in human and mouse OA cartilage, and Lrp5 deficiency in mice inhibited cartilage destruction. Treatment with IL-1β or Wnt decreased the level of Col2a1 and increased those of Mmp3 or Mmp13, whereas Lrp5 knockdown ameliorated these effects. In addition, we found that the functions of LRP5 in arthritic cartilage were subject to transcriptional activation by β-catenin. Moreover, Lrp5⁻/⁻ and Lrp5fl/fl;Col2a1-cre mice exhibited decreased cartilage destruction (and related changes in gene expression) in response to experimental OA.Our findings indicate that LRP5 (but not LRP6) plays an essential role in Wnt/β-catenin-signaling-mediated OA cartilage destruction in part by regulating the expression levels of type II collagen, MMP3, and MMP13. | | 24479426
 |
Prostaglandin E2 (PGE2) exerts biphasic effects on human tendon stem cells.
Zhang, J; Wang, JH
PloS one
9
e87706
2014
Show Abstract
Prostaglandin E2 (PGE2) has been reported to exert different effects on tissues at low and high levels. In the present study, cell culture experiments were performed to determine the potential biphasic effects of PGE2 on human tendon stem/progenitor cells (hTSCs). After treatment with PGE2, hTSC proliferation, stemness, and differentiation were analyzed. We found that high concentrations of PGE2 (greater than 1 ng/ml) decreased cell proliferation and induced non-tenocyte differentiation. However, at lower concentrations (less than 1 ng/ml), PGE2 markedly enhanced hTSC proliferation. The expression levels of stem cell marker genes, specifically SSEA-4 and Stro-1, were more extensive in hTSCs treated with low concentrations of PGE2 than in cells treated with high levels of PGE2. Moreover, high levels of PGE2 induced hTSCs to differentiate aberrantly into non-tenocytes, which was evident by the high levels of PPARγ, collagen type II, and osteocalcin expression in hTSCs treated with PGE2 at concentrations greater than 1 ng/ml. The findings of this study reveal that PGE2 can exhibit biphasic effects on hTSCs, indicating that while high PGE2 concentrations may be detrimental to tendons, low levels of PGE2 may play a vital role in the maintenance of tendon homeostasis in vivo. | | 24504456
 |
Increased mandibular condylar growth in mice with estrogen receptor beta deficiency.
Kamiya, Y; Chen, J; Xu, M; Utreja, A; Choi, T; Drissi, H; Wadhwa, S
Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research
28
1127-34
2013
Show Abstract
Temporomandibular joint (TMJ) disorders predominantly afflict women of childbearing age, suggesting a role for female hormones in the disease process. In long bones, estrogen acting via estrogen receptor beta (ERβ) inhibits axial skeletal growth in female mice. However, the role of ERβ in the mandibular condyle is largely unknown. We hypothesize that female ERβ-deficient mice will have increased mandibular condylar growth compared to wild-type (WT) female mice. This study examined female 7-day-old, 49-day-old, and 120-day-old WT and ERβ knockout (KO) mice. There was a significant increase in mandibular condylar cartilage thickness as a result of an increased number of cells, in the 49-day-old and 120-day-old female ERβ KO compared with WT controls. Analysis in 49-day-old female ERβ KO mice revealed a significant increase in collagen type X, parathyroid hormone-related protein (Pthrp), and osteoprotegerin gene expression and a significant decrease in receptor activator for nuclear factor κ B ligand (Rankl) and Indian hedgehog (Ihh) gene expression, compared with WT controls. Subchondral bone analysis revealed a significant increase in total condylar volume and a decrease in the number of osteoclasts in the 49-day-old ERβ KO compared with WT female mice. There was no difference in cell proliferation in condylar cartilage between the genotypes. However, there were differences in the expression of proteins that regulate the cell cycle; we found a decrease in the expression of Tieg1 and p57 in the mandibular condylar cartilage from ERβ KO mice compared with WT mice. Taken together, our results suggest that ERβ deficiency increases condylar growth in female mice by inhibiting the turnover of fibrocartilage. | | 23197372
 |
Repair of a chondral defect using a cell free scaffold in a young patient--a case report of successful scaffold transformation and colonisation.
Schüettler, KF; Struewer, J; Rominger, MB; Rexin, P; Efe, T
BMC surgery
13
11
2013
Show Abstract
Chondral defects of the articular surface are a common condition that can lead to osteoarthritis if not treated. Therapy of this condition is a topic of constant debate and a variety of chondral repair strategies are currently used. One strategy involves implantation of a cell-free matrix of type I collagen (COL1), to provide a scaffold for chondrocyte migration and proliferation and extracellular matrix production. Although several studies have suggested that chondrocytes can move, to the best of our knowledge there is still no proof of chondrocyte occurrence in a former cell-free scaffold for articular cartilage repair in humans.An 18-year-old male patient underwent arthroscopic surgery of the knee for patellar instability and a chondral defect of the femoral condyle. Clinical outcome scores were recorded pre-operatively, after 6 weeks and after 6, 12, 24 and 36 months. MRI was recorded after 6 weeks and after 6, 12, 24 and 36 months postoperatively. At 42 months after implantation of a cell-free type I collagen matrix and reconstruction of the medial patellofemoral ligament, the patient was again treated arthroscopically for a tear of the medial meniscus of the same knee. A biopsy of the previous chondral defect was taken during arthroscopy for histological examination.In addition to good clinical and radiological results reported for cell-free scaffolds for cartilage repair in several other studies, transformation of the scaffold could be observed during re-arthroscopy for the meniscal tear. Histological examination of the specimen revealed articular cartilage with vital chondrocytes and a strong staining reaction for type II collagen (COL II), but no reaction for type I collagen staining. This might indicate a complete transformation of the scaffold and supports the theory that cell free scaffolds could support cell migration. Although the cell source remains unclear, migrating chondrocytes from the periphery remain a possibility. | | 23590134
 |
Basic fibroblast growth factor supports expansion of mouse compact bone-derived mesenchymal stem cells (MSCs) and regeneration of bone from MSC in vivo.
Eiki Yamachika,Hidetsugu Tsujigiwa,Masakazu Matsubara,Yasuhisa Hirata,Kenichiro Kita,Kiyofumi Takabatake,Nobuyoshi Mizukawa,Yoshihiro Kaneda,Hitoshi Nagatsuka,Seiji Iida
Journal of molecular histology
43
2011
Show Abstract
Some progress has been made in development of methods to regenerate bone from cultured cells, however no method is put to practical use. Here, we developed methods to isolate, purify, and expand mesenchymal stem cells (MSCs) from mouse compact bone that may be used to regenerate bone in vivo. These cells were maintained in long-term culture and were capable of differentiating along multiple lineages, including chondrocyte, osteocyte, and adipocyte trajectories. We used standard cell isolation and culture methods to establish cell cultures from mouse compact bone and bone marrow. Cultures were grown in four distinct media to determine the optimal composition of culture medium for bone-derived MSCs. Putative MSCs were subjected to flow cytometry, alkaline phosphatase assays, immunohistochemical staining, and several differentiation assays to assess cell identity, protein expression, and developmental potential. Finally, we used an in vivo bone formation assay to determine whether putative MSCs were capable of regenerating bone. We found that compact bone of mice was a better source of MCSs than the bone marrow, that growth in plastic flasks served to purify MSCs from hematopoietic cells, and that MSCs grown in basic fibroblast growth factor (bFGF)-conditioned medium were, based on multiple criteria, superior to those grown in leukemia inhibitory factor-conditioned medium. Moreover, we found that the MSCs isolated from compact bone and grown in bFGF-conditioned medium were capable of supporting bone formation in vivo. The methods and results described here have implications for understanding MSC biology and for clinical purpose. | | 22203245
 |
Structural and functional analysis of intra-articular interzone tissue in axolotl salamanders.
Cosden-Decker, RS; Bickett, MM; Lattermann, C; MacLeod, JN
Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society
20
1347-56
2011
Show Abstract
Knowledge of mechanisms directing diarthrodial joint development may be useful in understanding joint pathologies and identifying new therapies. We have previously established that axolotl salamanders can fully repair large articular cartilage lesions, which may be due to the presence of an interzone-like tissue in the intra-articular space. Study objectives were to further characterize axolotl diarthrodial joint structure and determine the differentiation potential of interzone-like tissue in a skeletal microenvironment.Diarthrodial joint morphology and expression of aggrecan, brother of CDO (BOC), type I collagen, type II collagen, and growth/differentiation factor 5 (GDF5) were examined in femorotibial joints of sexually mature (greater than 12 months) axolotls. Joint tissue cellularity was evaluated in individuals from 2 to 24 months of age. Chondrogenic potential of the interzone was evaluated by placing interzone-like tissue into 4 mm tibial defects.Cavitation reached completion in the femoroacetabular and humeroradial joints, but an interzone-like tissue was retained in the intra-articular space of distal limb joints. Joint tissue cellularity decreased to 7 months of age and then remained stable. Gene expression patterns of joint markers are broadly similar in developing mammals and mature axolotls. When interzone-like tissue was transplanted into critical size skeletal defects, an accessory joint developed within the defect site.These experiments indicate that mature axolotl diarthrodial joints are phenotypically similar to developing synovial joints in mammals. Generation of an accessory joint by interzone-like tissue suggests multipotent cellular differentiation potential similar to that of interzone cells in the mammalian fetus. The data support the axolotl as a novel vertebrate model for joint development and repair. | Immunohistochemistry | 22800772
 |
Uniaxial mechanical strain modulates the differentiation of neural crest stem cells into smooth muscle lineage on micropatterned surfaces.
Li, X; Chu, J; Wang, A; Zhu, Y; Chu, WK; Yang, L; Li, S
PloS one
6
e26029
2010
Show Abstract
Neural crest stem cells (NCSCs) play an important role in the development and represent a valuable cell source for tissue engineering. However, how mechanical factors in vivo regulate NCSC differentiation is not understood. Here NCSCs were derived from induced pluripotent stem cells and used as a model to determine whether vascular mechanical strain modulates the differentiation of NCSCs into smooth muscle (SM) lineage. NCSCs were cultured on micropatterned membranes to mimic the organization of smooth muscle cells (SMCs), and subjected to cyclic uniaxial strain. Mechanical strain enhanced NCSC proliferation and ERK2 phosphorylation. In addition, mechanical strain induced contractile marker calponin-1 within 2 days and slightly induced SM myosin within 5 days. On the other hand, mechanical strain suppressed the differentiation of NCSCs into Schwann cells. The induction of calponin-1 by mechanical strain was inhibited by neural induction medium but further enhanced by TGF-β. For NCSCs pre-treated with TGF-β, mechanical strain induced the gene expression of both calponin-1 and SM myosin. Our results demonstrated that mechanical strain regulates the differentiation of NCSCs in a manner dependent on biochemical factors and the differentiation stage of NCSCs. Understanding the mechanical regulation of NCSC differentiation will shed light on the development and remodeling of vascular tissues, and how transplanted NCSCs respond to mechanical factors. Full Text Article | | 22016804
 |
Articular Cartilage Regeneration With Autologous Peripheral Blood Progenitor Cells and Hyaluronic Acid After Arthroscopic Subchondral Drilling: A Report of 5 Cases With Histology.
Saw KY, Anz A, Merican S, Tay YG, Ragavanaidu K, Jee CS, McGuire DA
Arthroscopy
2010
Show Abstract
PURPOSE: The purpose of this study was to evaluate the quality of articular cartilage regeneration after arthroscopic subchondral drilling followed by postoperative intraarticular injections of autologous peripheral blood progenitor cells (PBPCs) in combination with hyaluronic acid (HA).METHODS: Five patients underwent second-look arthroscopy with chondral core biopsy. These 5 patients are part of a larger pilot study in which 180 patients with International Cartilage Repair Society grade III and IV lesions of the knee joint underwent arthroscopic subchondral drilling followed by postoperative intra-articular injections. Continuous passive motion was used on the operated knee 2 hours per day for 4 weeks. Partial weight bearing was observed for the first 6 to 8 weeks. Autologous PBPCs were harvested 1 week after surgery. One week after surgery, 8 mL of the harvested PBPCs in combination with 2 mL of HA was injected intra-articularly into the operated knee. The remaining PBPCs were divided into vials and cryopreserved. A total of 5 weekly intra-articular injections were given.RESULTS: Second-look arthroscopy confirmed articular cartilage regeneration, and histologic sections showed features of hyaline cartilage. Apart from the minimal discomfort of PBPC harvesting and localized pain associated with the intra-articular injections, there were no other notable adverse reactions.CONCLUSIONS: Articular hyaline cartilage regeneration is possible with arthroscopic subchondral drilling followed by postoperative intraarticular injections of autologous PBPCs in combination with HA.LEVEL OF EVIDENCE: Level IV, therapeutic case series.Copyright © 2011 Arthroscopy Association of North America. Published by Elsevier Inc. All rights reserved. | | 21334844
 |