03-0197-00 MilliporeSMC™ Human SARS-CoV-2 S1 IgA Kit
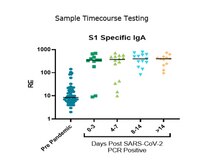
Assay mechanism includes proprietary elution and digital molecular counting steps that enable the SMC™ SARS-CoV-2 S1 IgA kit to detect antigen specific IgA in human serum and plasma at much lower levels compared to traditional immunoassays.
More>> Assay mechanism includes proprietary elution and digital molecular counting steps that enable the SMC™ SARS-CoV-2 S1 IgA kit to detect antigen specific IgA in human serum and plasma at much lower levels compared to traditional immunoassays. Less<<Recommended Products
Přehled
| Replacement Information |
|---|
Tabulka spec. kláve
| Key Applications |
|---|
| Single Molecule Counting (SMC®) |
| References |
|---|
| Biological Information |
|---|
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Storage and Shipping Information |
|---|
| Packaging Information | |
|---|---|
| Material Size | 96 well assay |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Katalogové číslo | GTIN |
| 03-0197-00 | 04065265648523 |
Documentation
Protocols
| Title |
|---|
| Protocol- 03-0197-00 |
| SMC Human SARS-CoV-2 S1 IgA Kit |
SMC™ Human SARS-CoV-2 S1 IgA Kit MSDS
| Title |
|---|