530656 Sigma-AldrichLTA4 Epoxide Hydrolase Inhibitor, ARM1 - Calbiochem
A cell-permeable, selective inhibitor of the epoxide hydrolase activity of leukotriene A4 hydrolase (Ki = 2.3 µM).
More>> A cell-permeable, selective inhibitor of the epoxide hydrolase activity of leukotriene A4 hydrolase (Ki = 2.3 µM). Less<<Synonyms: 4-(4-Benzylphenyl)-thiazol-2-amine, Leukotriene A4 Epoxide Hydrolase Inhibitor, LTA₄ Hydrolase Inhibitorq, LTA4H Inhibitor
Recommended Products
Přehled
| Replacement Information |
|---|
Tabulka spec. kláve
| Empirical Formula |
|---|
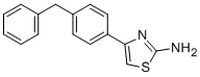
| C₁₆H₁₄N₂S |
| Description | |
|---|---|
| Overview | This product has been discontinued. A cell-permeable benzylphenyl-thiazolamine compound that acts as a selective inhibitor against the epoxide hydrolase activity of leukotriene A4 hydrolase (LTA4H; Ki = 2.3 µM) and inhibits A23187- (Cat. Nos. 100105 & 100106) induced leukotriene B4 (LTB4) synthesis in primary human polymorphonuclear neutrophil (PMN) cultures (IC50 = 0.5 µM). Does not affect LTA4H aminopeptidase activity toward the cleavage of Pro-Gly-pro even at a high concentratin of 100 µM. Binds to the hydrophobic pocket that accommodates LTA4 ω-end, which is distant from the aminopeptidase active site. |
| Catalogue Number | 530656 |
| Brand Family | Calbiochem® |
| Synonyms | 4-(4-Benzylphenyl)-thiazol-2-amine, Leukotriene A4 Epoxide Hydrolase Inhibitor, LTA₄ Hydrolase Inhibitorq, LTA4H Inhibitor |
| References | |
|---|---|
| References | Stsiapanava, A., et al. 2014. Proc. Natl. Acad. Sci. USA 111, 4227. |
| Product Information | |
|---|---|
| Form | Light beige powder |
| Hill Formula | C₁₆H₁₄N₂S |
| Chemical formula | C₁₆H₁₄N₂S |
| Reversible | Y |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | LTA4 |
| Purity | ≥98% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Katalogové číslo | GTIN |
| 530656 | 0 |
Documentation
LTA4 Epoxide Hydrolase Inhibitor, ARM1 - Calbiochem MSDS
| Title |
|---|
References
| Přehled odkazů |
|---|
| Stsiapanava, A., et al. 2014. Proc. Natl. Acad. Sci. USA 111, 4227. |
| Data Sheet | ||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Note that this data sheet is not lot-specific and is representative of the current specifications for this product. Please consult the vial label and the certificate of analysis for information on specific lots. Also note that shipping conditions may differ from storage conditions.
|