530588 Sigma-AldrichIκB Ubiquitination Inhibitor, GS143 - CAS 916232-21-8 - Calbiochem
A cell-permeable, selective inhibitor of E3 ligase complex SCFβTrCP1-mediated ubiquitination of phosphorylated IκBα (IC₅₀ = 5.2 µM).
More>> A cell-permeable, selective inhibitor of E3 ligase complex SCFβTrCP1-mediated ubiquitination of phosphorylated IκBα (IC₅₀ = 5.2 µM). Less<<Synonyms: 4-(3-Benzyl-4-(5-(2-fluoro-phenyl)-furan-2-ylmethylene)-5-oxo-4,5-dihydropyrazol-1-yl)-benzoic acid, 4-(3-Benzyl-4-((5-(2-fluorophenyl)furan-2-yl)methylene)-5-oxo-4,5-dihydro-1H-pyrazol-1-yl)benzoic acid, GS 143
Recommended Products
Přehled
| Replacement Information |
|---|
Tabulka spec. kláve
| CAS # | Empirical Formula |
|---|---|
| 916232-21-8 | C₂₈H₁₉FN₂O₄ |
Products
| Katalogové číslo | Balení | ks/bal. | |
|---|---|---|---|
| 5.30588.0001 | Skleněná láhev | 10 mg |
| References | |
|---|---|
| References | Hirose, K., et al. 2008. Biochem. Biophys. Res. Commun. 374, 507. Nakajima, H., et al. 2008. Biochem. Biophys. Res. Commun. 368, 1007. |
| Product Information | |
|---|---|
| CAS number | 916232-21-8 |
| Form | Dark red powder |
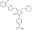
| Hill Formula | C₂₈H₁₉FN₂O₄ |
| Chemical formula | C₂₈H₁₉FN₂O₄ |
| Structure formula Image | |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | phosphorylated IκBα |
| Purity | ≥97% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Katalogové číslo | GTIN |
| 5.30588.0001 | 04055977260939 |
Documentation
IκB Ubiquitination Inhibitor, GS143 - CAS 916232-21-8 - Calbiochem MSDS
| Title |
|---|
References
| Přehled odkazů |
|---|
| Hirose, K., et al. 2008. Biochem. Biophys. Res. Commun. 374, 507. Nakajima, H., et al. 2008. Biochem. Biophys. Res. Commun. 368, 1007. |