531167 Sigma-AldrichGSK-3 Inhibitor XXIX, CHIR98014 - CAS 252935-94-7 - Calbiochem
A cell-permeable, highly potent, ATP-competitive, reversible inhibitor of both GSK-3α and β (IC₅₀ = 650 and 580 pM, respectively).
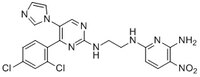
More>> A cell-permeable, highly potent, ATP-competitive, reversible inhibitor of both GSK-3α and β (IC₅₀ = 650 and 580 pM, respectively). Less<<Synonyms: N²-(2-(4-(2,4-Dichlorophenyl)-5-(1H-imidazol-1-yl)pyrimidin-2-ylamino)ethyl)-5-nitropyridine-2,6-diamine, N⁶-(2-((4-(2,4-Dichlorophenyl)-5-(1H-imidazol-1-yl)-2-pyrimidinyl)amino}ethyl)-3-nitro-2,6-pyridinediamine, CHIR-98014, CT-98014
Recommended Products
Přehled
| Replacement Information |
|---|
Tabulka spec. kláve
| CAS # | Empirical Formula |
|---|---|
| 252935-94-7 | C₂₀H₁₇Cl₂N₉O₂ |
Products
| Katalogové číslo | Balení | ks/bal. | |
|---|---|---|---|
| 5.31167.0001 | Skleněná láhev | 5 mg |
| Product Information | |
|---|---|
| CAS number | 252935-94-7 |
| Form | Yellow powder |
| Hill Formula | C₂₀H₁₇Cl₂N₉O₂ |
| Chemical formula | C₂₀H₁₇Cl₂N₉O₂ |
| Reversible | Y |
| Quality Level | MQ100 |
| Applications |
|---|
| Biological Information | |
|---|---|
| Primary Target | GSK-3 α & β |
| Primary Target IC<sub>50</sub> | 650 and 580 pM for GSK-3&alpha |
| Primary Target K<sub>i</sub> | 870 pM for human GSK-3&beta |
| Purity | ≥95% by HPLC |
| Physicochemical Information | |
|---|---|
| Cell permeable | Y |
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information | |
|---|---|
| Packaged under inert gas | Packaged under inert gas |
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Katalogové číslo | GTIN |
| 5.31167.0001 | 04055977283150 |
Documentation
GSK-3 Inhibitor XXIX, CHIR98014 - CAS 252935-94-7 - Calbiochem MSDS
| Title |
|---|
References
| Přehled odkazů |
|---|
| Grigoryan, T., et al. 2013. Proc. Natl. Acad. Sci. USA 110, 18174. Lian, X., et al. 2012. Proc. Natl. Acad. Sci. USA 109, E1848. Selenica, M.L., et al. 2007. Br. J. Pharmacol. 152, 959. Ring, D.B., et al. 2003. Diabetes. 52, 588. Nikoulina, S.E., et al. 2002. Diabetes. 51, 2190. |
Technical Info
| Title |
|---|
| Characterization of Estrogen Receptor α Phosphorylation Sites in Breast Cancer Tissue Using the SNAP i.d® 2.0 System |
| White Paper: Further considerations of antibody validation and usage. |