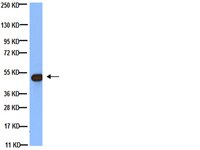
Shifts in temperature within the physiologic range modify strand-specific expression of select human microRNAs.
Potla, R; Singh, IS; Atamas, SP; Hasday, JD
RNA (New York, N.Y.)
21
1261-73
2015
Zobrazit abstrakt
Previous studies have revealed that clinically relevant changes in temperature modify clinically relevant gene expression profiles through transcriptional regulation. Temperature dependence of post-transcriptional regulation, specifically, through expression of miRNAs has been less studied. We comprehensively analyzed the effect of 24 h exposure to 32°C or 39.5°C on miRNA expression profile in primary cultured human small airway epithelial cells (hSAECs) and its impact on expression of a targeted protein, protein kinase C α (PKCα). Using microarray, and solution hybridization-based nCounter assays, with confirmation by quantitative RT-PCR, we found significant temperature-dependent changes in expression level of only five mature human miRNAs, representing only 1% of detected miRNAs. Four of these five miRNAs are the less abundant passenger (star) strands. They exhibited a similar pattern of increased expression at 32°C and reduced expression at 39.5°C relative to 37°C. As PKCα mRNA has multiple potential binding sites for three of these miRNAs, we analyzed PKCα protein expression in HEK 293T cells and hSAECs. PKCα protein levels were lowest at 32°C and highest at 39.5°C and specific miRNA inhibitors reduced these effects. Finally, we analyzed cell-cycle progression in hSAECs and found 32°C cells exhibited the greatest G1 to S transition, a process known to be inhibited by PKCα, and the effect was mitigated by specific miRNA inhibitors. These results demonstrate that exposure to clinically relevant hypothermia or hyperthermia modifies expression of a narrow subset of miRNAs and impacts expression of at least one signaling protein involved in multiple important cellular processes. | | | 26018549
 |
Zinc finger independent genome-wide binding of Sp2 potentiates recruitment of histone-fold protein Nf-y distinguishing it from Sp1 and Sp3.
Völkel, S; Stielow, B; Finkernagel, F; Stiewe, T; Nist, A; Suske, G
PLoS genetics
11
e1005102
2015
Zobrazit abstrakt
Transcription factors are grouped into families based on sequence similarity within functional domains, particularly DNA-binding domains. The Specificity proteins Sp1, Sp2 and Sp3 are paradigmatic of closely related transcription factors. They share amino-terminal glutamine-rich regions and a conserved carboxy-terminal zinc finger domain that can bind to GC rich motifs in vitro. All three Sp proteins are ubiquitously expressed; yet they carry out unique functions in vivo raising the question of how specificity is achieved. Crucially, it is unknown whether they bind to distinct genomic sites and, if so, how binding site selection is accomplished. In this study, we have examined the genomic binding patterns of Sp1, Sp2 and Sp3 in mouse embryonic fibroblasts by ChIP-seq. Sp1 and Sp3 essentially occupy the same promoters and localize to GC boxes. The genomic binding pattern of Sp2 is different; Sp2 primarily localizes at CCAAT motifs. Consistently, re-expression of Sp2 and Sp3 mutants in corresponding knockout MEFs revealed strikingly different modes of genomic binding site selection. Most significantly, while the zinc fingers dictate genomic binding of Sp3, they are completely dispensable for binding of Sp2. Instead, the glutamine-rich amino-terminal region is sufficient for recruitment of Sp2 to its target promoters in vivo. We have identified the trimeric histone-fold CCAAT box binding transcription factor Nf-y as the major partner for Sp2-chromatin interaction. Nf-y is critical for recruitment of Sp2 to co-occupied regulatory elements. Equally, Sp2 potentiates binding of Nf-y to shared sites indicating the existence of an extensive Sp2-Nf-y interaction network. Our results unveil strikingly different recruitment mechanisms of Sp1/Sp2/Sp3 transcription factor members uncovering an unexpected layer of complexity in their binding to chromatin in vivo. | | | 25793500
 |
Type-1 interferons contribute to oxygen glucose deprivation induced neuro-inflammation in BE(2)M17 human neuroblastoma cells.
Minter, MR; Zhang, M; Ates, RC; Taylor, JM; Crack, PJ
Journal of neuroinflammation
11
43
2014
Zobrazit abstrakt
Hypoxic-ischaemic injuries such as stroke and traumatic brain injury exhibit features of a distinct neuro-inflammatory response in the hours and days post-injury. Microglial activation, elevated pro-inflammatory cytokines and macrophage infiltration contribute to core tissue damage and contribute to secondary injury within a region termed the penumbra. Type-1 interferons (IFNs) are a super-family of pleiotropic cytokines that regulate pro-inflammatory gene transcription via the classical Jak/Stat pathway; however their role in hypoxia-ischaemia and central nervous system neuro-inflammation remains unknown. Using an in vitro approach, this study investigated the role of type-1 IFN signalling in an inflammatory setting induced by oxygen glucose deprivation (OGD).Human BE(2)M17 neuroblastoma cells or cells expressing a type-1 interferon-α receptor 1 (IFNAR1) shRNA or negative control shRNA knockdown construct were subjected to 4.5 h OGD and a time-course reperfusion period (0 to 24 h). Q-PCR was used to evaluate IFNα, IFNβ, IL-1β, IL-6 and TNF-α cytokine expression levels. Phosphorylation of signal transducers and activators of transcription (STAT)-1, STAT-3 and cleavage of caspase-3 was detected by western blot analysis. Post-OGD cellular viability was measured using a MTT assay.Elevated IFNα and IFNβ expression was detected during reperfusion post-OGD in parental M17 cells. This correlated with enhanced phosphorylation of STAT-1, a downstream type-1 IFN signalling mediator. Significantly, ablation of type-1 IFN signalling, through IFNAR1 knockdown, reduced IFNα, IFNβ, IL-6 and TNF-α expression in response to OGD. In addition, MTT assay confirmed the IFNAR1 knockdown cells were protected against OGD compared to negative control cells with reduced pro-apoptotic cleaved caspase-3 levels.This study confirms a role for type-1 IFN signalling in the neuro-inflammatory response following OGD in vitro and suggests its modulation through therapeutic blockade of IFNAR1 may be beneficial in reducing hypoxia-induced neuro-inflammation. | | | 24602263
 |
Contactin-1 regulates myelination and nodal/paranodal domain organization in the central nervous system.
Çolakoğlu, G; Bergstrom-Tyrberg, U; Berglund, EO; Ranscht, B
Proceedings of the National Academy of Sciences of the United States of America
111
E394-403
2014
Zobrazit abstrakt
Myelin, a multilayered membrane sheath formed by oligodendrocytes around axons in the CNS, enables rapid nerve impulse conduction and sustains neuronal health. The signals exchanged between axons and oligodendrocytes in myelin remain to be fully elucidated. Here we provide genetic evidence for multiple and critical functions of Contactin-1 in central myelin. We document dynamic Contactin-1 expression on oligodendrocytes in vivo, and progressive accumulation at nodes of Ranvier and paranodes during postnatal mouse development. Nodal and paranodal expression stabilized in mature myelin, but overall membranous expression diminished. Contactin-1-deficiency disrupted paranodal junction formation as evidenced by loss of Caspr, mislocalized potassium Kv1.2 channels, and abnormal myelin terminal loops. Reduced numbers and impaired maturation of sodium channel clusters accompanied this phenotype. Histological, electron microscopic, and biochemical analyses uncovered significant hypomyelination in Contactin-1-deficient central nerves, with up to 60% myelin loss. Oligodendrocytes were present in normal numbers, albeit a minor population of neuronal/glial antigen 2-positive (NG2(+)) progenitors lagged in maturation by postnatal day 18, when the mouse null mutation was lethal. Major contributing factors to hypomyelination were defects in the generation and organization of myelin membranes, as judged by electron microscopy and quantitative analysis of oligodendrocyte processes labeled by GFP transgenically expressed from the proteolipid protein promoter. These data reveal that Contactin-1 regulates both myelin formation and organization of nodal and paranodal domains in the CNS. These multiple roles distinguish central Contactin-1 functions from its specific role at paranodes in the periphery, and emphasize mechanistic differences in central and peripheral myelination. | | | 24385581
 |
The sphingolipid receptor S1PR2 is a receptor for Nogo-a repressing synaptic plasticity.
Kempf, A; Tews, B; Arzt, ME; Weinmann, O; Obermair, FJ; Pernet, V; Zagrebelsky, M; Delekate, A; Iobbi, C; Zemmar, A; Ristic, Z; Gullo, M; Spies, P; Dodd, D; Gygax, D; Korte, M; Schwab, ME
PLoS biology
12
e1001763
2014
Zobrazit abstrakt
Nogo-A is a membrane protein of the central nervous system (CNS) restricting neurite growth and synaptic plasticity via two extracellular domains: Nogo-66 and Nogo-A-Δ20. Receptors transducing Nogo-A-Δ20 signaling remained elusive so far. Here we identify the G protein-coupled receptor (GPCR) sphingosine 1-phosphate receptor 2 (S1PR2) as a Nogo-A-Δ20-specific receptor. Nogo-A-Δ20 binds S1PR2 on sites distinct from the pocket of the sphingolipid sphingosine 1-phosphate (S1P) and signals via the G protein G13, the Rho GEF LARG, and RhoA. Deleting or blocking S1PR2 counteracts Nogo-A-Δ20- and myelin-mediated inhibition of neurite outgrowth and cell spreading. Blockade of S1PR2 strongly enhances long-term potentiation (LTP) in the hippocampus of wild-type but not Nogo-A(-/-) mice, indicating a repressor function of the Nogo-A/S1PR2 axis in synaptic plasticity. A similar increase in LTP was also observed in the motor cortex after S1PR2 blockade. We propose a novel signaling model in which a GPCR functions as a receptor for two structurally unrelated ligands, a membrane protein and a sphingolipid. Elucidating Nogo-A/S1PR2 signaling platforms will provide new insights into regulation of synaptic plasticity. | | | 24453941
 |
Stress-induced localization of HSPA6 (HSP70B') and HSPA1A (HSP70-1) proteins to centrioles in human neuronal cells.
Khalouei, S; Chow, AM; Brown, IR
Cell stress & chaperones
19
321-7
2014
Zobrazit abstrakt
The localization of yellow fluorescent protein (YFP)-tagged HSP70 proteins was employed to identify stress-sensitive sites in human neurons following temperature elevation. Stable lines of human SH-SY5Y neuronal cells were established that expressed YFP-tagged protein products of the human inducible HSP70 genes HSPA6 (HSP70B') and HSPA1A (HSP70-1). Following a brief period of thermal stress, YFP-tagged HSPA6 and HSPA1A rapidly appeared at centrioles in the cytoplasm of human neuronal cells, with HSPA6 demonstrating a more prolonged signal compared to HSPA1A. Each centriole is composed of a distal end and a proximal end, the latter linking the centriole doublet. The YFP-tagged HSP70 proteins targeted the proximal end of centrioles (identified by γ-tubulin marker) rather than the distal end (centrin marker). Centrioles play key roles in cellular polarity and migration during neuronal differentiation. The proximal end of the centriole, which is involved in centriole stabilization, may be stress-sensitive in post-mitotic, differentiating human neurons. | Western Blotting | | 24061851
 |
Nucleosome positioning and histone modifications define relationships between regulatory elements and nearby gene expression in breast epithelial cells.
Rhie, SK; Hazelett, DJ; Coetzee, SG; Yan, C; Noushmehr, H; Coetzee, GA
BMC genomics
15
331
2014
Zobrazit abstrakt
The precise nature of how cell type specific chromatin structures at enhancer sites affect gene expression is largely unknown. Here we identified cell type specific enhancers coupled with gene expression in two different types of breast epithelial cells, HMEC (normal breast epithelial cells) and MDAMB231 (triple negative breast cancer cell line).Enhancers were defined by modified neighboring histones [using chromatin immunoprecipitation followed by sequencing (ChIP-seq)] and nucleosome depletion [using formaldehyde-assisted isolation of regulatory elements followed by sequencing (FAIRE-seq)]. Histone modifications at enhancers were related to the expression levels of nearby genes up to 750 kb away. These expression levels were correlated with enhancer status (poised or active), defined by surrounding histone marks. Furthermore, about fifty percent of poised and active enhancers contained nucleosome-depleted regions. We also identified response element motifs enriched at these enhancer sites that revealed key transcription factors (e.g. TP63) likely involved in regulating breast epithelial enhancer-mediated gene expression. By utilizing expression data, potential target genes of more than 600 active enhancers were identified. These genes were involved in proteolysis, epidermis development, cell adhesion, mitosis, cell cycle, and DNA replication.These findings facilitate the understanding of epigenetic regulation specifically, such as the relationships between regulatory elements and gene expression and generally, how breast epithelial cellular phenotypes are determined by cell type specific enhancers. | Western Blotting | | 24885402
 |
Comparative analysis of single and combined APP/APLP knockouts reveals reduced spine density in APP-KO mice that is prevented by APPsα expression.
Weyer, SW; Zagrebelsky, M; Herrmann, U; Hick, M; Ganss, L; Gobbert, J; Gruber, M; Altmann, C; Korte, M; Deller, T; Müller, UC
Acta neuropathologica communications
2
36
2014
Zobrazit abstrakt
Synaptic dysfunction and synapse loss are key features of Alzheimer's pathogenesis. Previously, we showed an essential function of APP and APLP2 for synaptic plasticity, learning and memory. Here, we used organotypic hippocampal cultures to investigate the specific role(s) of APP family members and their fragments for dendritic complexity and spine formation of principal neurons within the hippocampus. Whereas CA1 neurons from APLP1-KO or APLP2-KO mice showed normal neuronal morphology and spine density, APP-KO mice revealed a highly reduced dendritic complexity in mid-apical dendrites. Despite unaltered morphology of APLP2-KO neurons, combined APP/APLP2-DKO mutants showed an additional branching defect in proximal apical dendrites, indicating redundancy and a combined function of APP and APLP2 for dendritic architecture. Remarkably, APP-KO neurons showed a pronounced decrease in spine density and reductions in the number of mushroom spines. No further decrease in spine density, however, was detectable in APP/APLP2-DKO mice. Mechanistically, using APPsα-KI mice lacking transmembrane APP and expressing solely the secreted APPsα fragment we demonstrate that APPsα expression alone is sufficient to prevent the defects in spine density observed in APP-KO mice. Collectively, these studies reveal a combined role of APP and APLP2 for dendritic architecture and a unique function of secreted APPs for spine density. | | | 24684730
 |
Localization of heat shock proteins in cerebral cortical cultures following induction by celastrol.
Chow, AM; Tang, DW; Hanif, A; Brown, IR
Cell stress & chaperones
19
845-51
2014
Zobrazit abstrakt
Hsp70, Hsp32, and Hsp27 were induced by celastrol in rat cerebral cortical cultures at dosages that did not affect cell viability. Pronounced differences were observed in the cellular localization of these heat shock proteins in cell types of cerebral cortical cultures. Celastrol-induced Hsp70 localized to the cell body and cellular processes of neurons that were identified by neuron-specific βIII-tubulin. Hsp70 was not detected in adjacent GFAP-positive glial cells that demonstrated a strong signal for Hsp27 and Hsp32 in both glial cell bodies and cellular processes. Cells in the cerebral cortex region of the brain are selectively impacted during the progression of Alzheimer's disease which is a "protein misfolding disorder." Heat shock proteins provide a line of defense against misfolded, aggregation-prone proteins. Celastrol is a potential agent to counter this neurodegenerative disorder as recent evidence indicates that in vivo administration of celastrol in a transgenic model of Alzheimer's reduces an important neuropathological hallmark of this disease, namely, amyloid beta pathology that involves protein aggregation. | | | 24700193
 |
Control of cultured human cells with femtosecond laser ablated patterns on steel and plastic surfaces.
Nuutinen, T; Silvennoinen, M; Päiväsaari, K; Vahimaa, P
Biomedical microdevices
15
279-88
2013
Zobrazit abstrakt
The purpose of the present study is to explore topographical patterns produced with femtosecond laser pulses as a means of controlling the behaviour of living human cells (U2OS) on stainless steel surfaces and on negative plastic imprints (polycarbonate). The results show that the patterns on both types of material strongly affect cell behaviour and are particularly powerful in controlling cell spreading/elongation, localization and orientation. Analysis by fluorescence and scanning electron microscopy shows that on periodic 1D grating structures, cells and cell nuclei are highly elongated and aligned, whereas on periodic 2D grid structures, cell spreading and shape is affected. The results also show that the density and morphology of the cells can be affected. This was observed particularly on pseudo-periodic, coral-like structures which clearly inhibited cell growth. The results suggest that these patterns could be used in a variety of applications among the fields of clinical research and implant design, as well as in diagnosis and in cell and drug research. Furthermore, this article highlights the noteworthy aspects and the unique strengths of the technique and proposes directions for further research. | | | 23179464
 |