569388 Sigma-AldrichAnti-STAT3 Rabbit pAb
Recommended Products
Přehled
| Replacement Information |
|---|
Tabulka spec. kláve
| Host |
|---|
| Rb |
| Description | |
|---|---|
| Overview | This product has been discontinued. We are offering Anti-Stat3 Antibody (Cat. No. 06-596) as a possible alternative. Please read the alternative product documentation carefully and contact technical service if you need additional information. |
| Catalogue Number | 569388 |
| Brand Family | Calbiochem® |
| Synonyms | Anti-Signal Transducer and Activator of Transcription 3 |
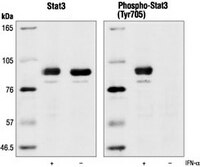
| Application Data |  Detection of human STAT3 by immunoblotting. Samples: Whole cell lysate from HeLa cell treated with IFN-α (lane 1) or left untreated (lane 2). Primary antibody: Anti-STAT3 Rabbit pAb (Cat. No. 569388) (1:1000). Detection: chemiluminescence. |
| Product Information | |
|---|---|
| Form | Liquid |
| Formulation | In 150 mM NaCl, 10 mM HEPES, 50% glycerol, 0.01% BSA, pH 7.5. |
| Preservative | None |
| Quality Level | MQ100 |
| Biological Information | |
|---|---|
| Immunogen | a synthetic peptide corresponding to amino acids surrounding Tyr⁷⁰⁵ of mouse STAT3 |
| Immunogen | Mouse |
| Host | Rabbit |
| Isotype | IgG |
| Physicochemical Information |
|---|
| Dimensions |
|---|
| Materials Information |
|---|
| Toxicological Information |
|---|
| Safety Information according to GHS |
|---|
| Safety Information |
|---|
| Product Usage Statements |
|---|
| Packaging Information |
|---|
| Transport Information |
|---|
| Supplemental Information |
|---|
| Specifications |
|---|
| Global Trade Item Number | |
|---|---|
| Katalogové číslo | GTIN |
| 569388 | 0 |
Documentation
Anti-STAT3 Rabbit pAb Certificates of Analysis
| Title | Lot Number |
|---|---|
| 569388 |
References
| Přehled odkazů |
|---|
| David, M., et al. 1995. Science 269, 1721. Ihle, J.N. 1995. Nature 377, 591. Wen, Z., et al. 1995. Cell 82, 241. Darnell, J.E., Jr., et al. 1994. Science 264, 1415. Ihle, J.N., et al. 1994. Trends Biochem. Sci. 19, 222. |