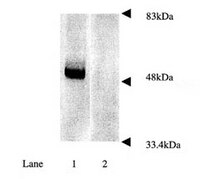
Nilotinib-induced autophagic changes increase endogenous parkin level and ubiquitination, leading to amyloid clearance.
Lonskaya, I; Hebron, ML; Desforges, NM; Schachter, JB; Moussa, CE
Journal of molecular medicine (Berlin, Germany)
92
373-86
2014
Zobrazit abstrakt
Alzheimer's disease (AD) is a neurodegenerative disorder associated with amyloid accumulation and autophagic changes. Parkin is an E3 ubiquitin ligase involved in proteasomal and autophagic clearance. We previously demonstrated decreased parkin solubility and interaction with the key autophagy enzyme beclin-1 in AD, but tyrosine kinase inhibition restored parkin-beclin-1 interaction. In the current studies, we determined the mechanisms of nilotinib-induced parkin-beclin-1 interaction, which leads to amyloid clearance. Nilotinib increased endogenous parkin levels and ubiquitination, which may enhance parkin recycling via the proteasome, leading to increased activity and interaction with beclin-1. Parkin solubility was decreased and autophagy was altered in amyloid expressing mice, suggesting that amyloid stress affects parkin stability, leading to failure of protein clearance via the lysosome. Isolation of autophagic vacuoles revealed amyloid and parkin accumulation in autophagic compartments but nilotinib decreased insoluble parkin levels and facilitated amyloid deposition into lysosomes in wild type, but not parkin(-/-) mice, further underscoring an essential role for endogenous parkin in amyloid clearance. These results suggest that nilotinib boosts the autophagic machinery, leading to increased level of endogenous parkin that undergoes ubiquitination and interacts with beclin-1 to facilitate amyloid clearance. These data suggest that nilotinib-mediated autophagic changes may trigger parkin response via increased protein levels, providing a therapeutic strategy to reduce Aβ and Tau in AD.Parkin solubility (stability) is decreased in AD and APP transgenic mice. Nilotinib-induced autophagic changes increase endogenous parkin level. Increased parkin level leads to ubiquitination and proteasomal recycling. Re-cycling decreases insoluble parkin and increases parkin-beclin-1 interaction. Beclin-1-parkin interaction enhances amyloid clearance. | | 24337465
 |
Increasing the Coding Potential of Genomes Through Alternative Splicing: The Case of PARK2 Gene.
La Cognata, V; Iemmolo, R; D'Agata, V; Scuderi, S; Drago, F; Zappia, M; Cavallaro, S
Current genomics
15
203-16
2014
Zobrazit abstrakt
The completion of the Human Genome Project aroused renewed interest in alternative splicing, an efficient and widespread mechanism that generates multiple protein isoforms from individual genes. Although our knowledge about alternative splicing is growing exponentially, its real impact on cellular life is still to be clarified. Connecting all splicing features (genes, splice transcripts, isoforms, and relative functions) may be useful to resolve this tangle. Herein, we will start from the case of a single gene, Parkinson protein 2, E3 ubiquitin protein ligase (PARK2), one of the largest in our genome. This gene is implicated in the pathogenesis of autosomal recessive juvenile Parkinsonism and it has been recently linked to cancer, leprosy, autism, type 2 diabetes mellitus and Alzheimer's disease. PARK2 primary transcript undergoes an extensive alternative splicing, which enhances transcriptomic diversification and protein diversity in tissues and cells. This review will provide an update of all human PARK2 alternative splice transcripts and isoforms presently known, and correlate them to those in rat and mouse, two common animal models for studying human disease genes. Alternative splicing relies upon a complex process that could be easily altered by both cis and trans-acting mutations. Although the contribution of PARK2 splicing in human disease remains to be fully explored, some evidences show disruption of this versatile form of genetic regulation may have pathological consequences. | | 24955028
 |
Decreased parkin solubility is associated with impairment of autophagy in the nigrostriatum of sporadic Parkinson's disease.
Lonskaya, I; Hebron, ML; Algarzae, NK; Desforges, N; Moussa, CE
Neuroscience
232
90-105
2013
Zobrazit abstrakt
Parkinson's disease (PD) is a motor disorder that involves death of dopaminergic neurons in the substantia nigra pars compacta. Parkin is an autosomal recessive gene that is mutated in early onset PD. We investigated the role of parkin and autophagic clearance in postmortem nigrostriatal tissues from 22 non-familial sporadic PD patients and 15 control samples. Parkin was insoluble with altered cytosolic expression in the nigrostriatum of sporadic PD. Parkin insolubility was associated with lack of degradation of ubiquitinated proteins and accumulation of α-Synuclein and parkin in autophagosomes, suggesting autophagic defects in PD. To test parkin's role in mediating autophagic clearance, we used lentiviral gene transfer to express human wild type or mutant parkin (T240R) with α-Synuclein in the rat striatum. Lentiviral expression of α-Synuclein led to accumulation of autophagic vacuoles, while co-expression of parkin with α-Synuclein facilitated autophagic clearance. Subcellular fractionation showed accumulation of α-Synuclein and tau hyper-phosphorylation (p-Tau) in autophagosomes in gene transfer models, similar to the effects observed in PD brains, but parkin expression led to protein deposition into lysosomes. However, parkin loss of function mutation did not affect autophagic clearance. Taken together, these data suggest that functional parkin regulates autophagosome clearance, while decreased parkin solubility may alter normal autophagy in sporadic PD. | | 23262240
 |
Tyrosine kinase inhibition increases functional parkin-Beclin-1 interaction and enhances amyloid clearance and cognitive performance.
Lonskaya, I; Hebron, ML; Desforges, NM; Franjie, A; Moussa, CE
EMBO molecular medicine
5
1247-62
2013
Zobrazit abstrakt
Tyrosine kinase inhibitors (TKIs) are effective therapies for leukaemia. Alzheimer is a neurodegenerative disease characterized by accumulation of β-amyloid (plaques) and hyper-phosphorylated Tau (tangles). Here we show that AD animals have high levels of insoluble parkin and decreased parkin-Beclin-1 interaction, while peripheral administration of TKIs, including Nilotinib and Bosutinib, increases soluble parkin leading to amyloid clearance and cognitive improvement. Blocking Beclin-1 expression with shRNA or parkin deletion prevents tyrosine kinase (TK) inhibition-induced amyloid clearance, suggesting that functional parkin-Beclin-1 interaction mediates amyloid degradation. Isolation of autophagic vacuoles (AVs) in AD mouse brain shows accumulation of parkin and amyloid, consistent with previous results in AD brains, while Bosutinib and Nilotinib increase parkin-Beclin-1 interaction and result in protein deposition in the lysosome. These data suggest that decreased parkin solubility impedes parkin-Beclin-1 interaction and amyloid clearance. We identified two FDA-approved anti-cancer drugs as potential treatment for AD. | Immunohistochemistry | 23737459
 |
Parkin expression profile in dopamine d3 receptor knock-out mice brains.
Velia D'Agata,Adriana Tiralongo,Alessandro Castorina,Gian Marco Leggio,Vincenzo Micale,Maria Luisa Carnazza,Filippo Drago
Neurochemical research
34
2009
Zobrazit abstrakt
Patients affected by autosomic recessive juvenile parkinsonism (ARJP) exhibit parkin gene mutations with brain decrease in dopamine D2/D3 binding sites. To date, there are no data indicating whether the reduction in dopamine D3 receptors (DRD3) may be associated with the expression of specific parkin variants. In the present study we investigated parkin expression profile in DRD3 knock-out mice brains. RT-PCR analysis was performed to assess qualitative changes in parkin isoforms' distribution pattern and in exons' expression both in wild type controls and dopamine D3 receptor's knock-out mice. Real-time PCR was performed to quantify single exons mRNA. Results demonstrated that exons 1, 2, 4, 6, 7, 8, were more expressed in wild type compared to dopamine D3 receptor KO mice brains while some other (3, 9, 10) were lower expressed. The expression levels of exons 5, 11 and 12 did not change in both animal groups. Our analysis was confirmed by western blot, which showed that parkin protein levels were influenced by the absence of DRD3. | | 18612813
 |
Parkin is expressed in vascular endothelial cells.
Wakako Tamo, Tadaatsu Imaizumi, Kunikazu Tanji, Hidemi Yoshida, Shingo Takanashi, Koichi Wakabayashi, Ryosuke Takahashi, Nobutaka Hattori, Kei Satoh
Neuroscience letters
419
199-201
2007
Zobrazit abstrakt
Mutations in the parkin gene are related with early-onset Parkinson's disease. Parkin is identified as an E3-ligase that combines target proteins with ubiquitin. alpha-Synuclein and synphilin-1 are substrates for E3-ligase activity of parkin and considered to be involved in the pathogenesis of Parkinson's disease. We previously demonstrated both alpha-synuclein and synphilin-1 are expressed in vascular endothelial cells (VEC). In the present study, we addressed possible expression of parkin in VEC. Parkin immunoreactivity was detected in vascular endothelial cells in postmortem human brain. Expressions of parkin mRNA and protein in human umbilical vein endothelial cells (HUVEC) were demonstrated by reverse-transcription polymerase-chain reaction (RT-PCR) and western blotting. Expression of parkin in HUVEC was not altered with tunicamycin treatment, which exerts unfolded protein stress on cells. We conclude that parkin is expressed in VEC, and that unfolded protein stress may not regulate its expression. | | 17481813
 |
Parkinson's disease transgenic mitochondrial cybrids generate Lewy inclusion bodies.
Trimmer, Patricia A, et al.
J. Neurochem., 88: 800-12 (2004)
2004
Zobrazit abstrakt
Many models of Parkinson's disease (PD) have succeeded in replicating dopaminergic neuron loss or alpha-synuclein aggregation but not the formation of classical Lewy bodies, the pathological hallmark of PD. Our cybrid model of sporadic PD was created by introducing the mitochondrial genes from PD patients into neuroblastoma cells that lack mitochondrial DNA. Previous studies using cybrids have shown that information encoded by mitochondrial DNA in patients contributes to many pathogenic features of sporadic PD. In this paper, we report the generation of fibrillar and vesicular inclusions in a long-term cybrid cell culture model that replicates the essential antigenic and structural features of Lewy bodies in PD brain without the need for exogenous protein expression or inhibition of mitochondrial or proteasomal function. The inclusions generated by PD cybrid cells stained with eosin, thioflavin S, and antibodies to alpha-synuclein, ubiquitin, parkin, synphilin-1, neurofilament, beta-tubulin, the proteasome, nitrotyrosine, and cytochrome c. Future studies of these cybrids will enable us to better understand how Lewy bodies form and what role they play in the pathogenesis of PD. | | 14756800
 |
Novel monoclonal antibodies demonstrate biochemical variation of brain parkin with age.
Pawlyk, AC; Giasson, BI; Sampathu, DM; Perez, FA; Lim, KL; Dawson, VL; Dawson, TM; Palmiter, RD; Trojanowski, JQ; Lee, VM
The Journal of biological chemistry
278
48120-8
2003
Zobrazit abstrakt
Autosomal recessive juvenile parkinsonism is a movement disorder associated with the degeneration of dopaminergic neurons in substantia nigra pars compacta. The loss of functional parkin caused by parkin gene mutations is the most common single cause of juvenile parkinsonism. Parkin has been shown to aid in protecting cells from endoplasmic reticulum and oxidative stressors presumably due to ubiquitin ligase activity of parkin that targets proteins for proteasomal degradation. However, studies on parkin have been impeded because of limited reagents specific for this protein. Here we report the generation and characterization of a panel of parkin-specific monoclonal antibodies. Biochemical analyses indicate that parkin is present only in the high salt-extractable fraction of mouse brain, whereas it is present in both the high salt-extractable and RIPA-resistant, SDS-extractable fraction in young human brain. Parkin is present at decreased levels in the high salt-extractable fraction and at increased levels in the SDS-extractable fraction from aged human brain. This shift in the extractability of parkin upon aging is seen in humans but not in mice, demonstrating species-specific differences in the biochemical characteristics of murine versus human parkin. Finally, by using these highly specific anti-parkin monoclonal antibodies, it was not possible to detect parkin in alpha-synuclein-containing lesions in alpha-synucleinopathies, thereby challenging prior inferences about the role of parkin in movement disorders other than autosomal recessive juvenile parkinsonism. | | 12972409
 |
Alterations in the common fragile site gene Parkin in ovarian and other cancers.
Denison, SR; Wang, F; Becker, NA; Schüle, B; Kock, N; Phillips, LA; Klein, C; Smith, DI
Oncogene
22
8370-8
2003
Zobrazit abstrakt
The cloning and characterization of the common fragile site (CFS) FRA6E (6q26) identified Parkin, the gene involved in the pathogenesis of many cases of juvenile, early-onset and, rarely, late-onset Parkinson's disease, as the third large gene to be localized within a large CFS. Initial analyses of Parkin indicated that in addition to playing a role in Parkinson's disease, it might also be involved in the development and/or progression of ovarian cancer. These analyses also indicated striking similarities among the large CFS-locus genes: fragile histidine triad gene (FHIT; 3p14.2), WW domain-containing oxidoreductase gene (WWOX; 16q23), and Parkin (6q26). Analyses of FHIT and WWOX in a variety of different cancer types have identified the presence of alternative transcripts with whole exon deletions. Interestingly, various whole exon duplications and deletions have been identified for Parkin in juvenile and early-onset Parkinson's patients. Therefore, we performed mutational/exon rearrangement analysis of Parkin in ovarian cancer cell lines and primary tumors. Four (66.7%) cell lines and four (18.2%) primary tumors were identified as being heterozygous for the duplication or deletion of a Parkin exon. Additionally, three of 23 (13.0%) nonovarian tumor-derived cell lines were also identified as having a duplication or deletion of one or more Parkin exons. Analysis of Parkin protein expression with antibodies revealed that most of the ovarian cancer cell lines and primary tumors had diminished or absent Parkin expression. While functional analyses have not yet been performed for Parkin, these data suggest that like FHIT and WWOX, Parkin may represent a tumor suppressor gene. | | 14614460
 |
Genomic organization and expression of parkin in Drosophila melanogaster.
Bae, YJ; Park, KS; Kang, SJ
Experimental & molecular medicine
35
393-402
2003
Zobrazit abstrakt
We report here the isolation, characterization on genomic structure and expression of the D. melanogaster homolog of human parkin. The 2,122 bp parkin gene sequence contains six exons that form a 1,449 bp transcript encoding a protein of 482 amino acids. 151 bp of 5' and 112 bp of 3' untranslated regions were identified by a combination of 5'-RACE/primer extension and 3'-RACE, respectively. The 5' UTR contains three transcription initiation sites. Neither a classical TATA nor a CAAT box was found in the putative promoter sequence. However, binding sites for AhR-Arnt, AP4, NF1 and GATA transcription factors were identified. Transient transfection analysis of the 5' UTR confirmed its promoter activity in HEK 293 cells and SH-SY5Y neuronal cells using a dual luciferase reporting system. The amino acid sequence of D. melanogaster Parkin exhibits 42%, 43% and 43% identity to that of human, mouse and rat, respectively, representing a 54 kDa protein band via western blot analysis. It shows a high degree of conservation in the Ubiquitin-like domain at the N-terminus (34%), the In-Between RING finger domains (IBR, 65-69%), and the RING finger domains at the C-terminus (56-57%). The expression pattern of D. melanogaster parkin varies during the developmental stages, with the highest expression in the adult stage as measured by competitive RT-PCR. From immunostainings of the embryo, D. melanogaster parkin was expressed slightly higher in the central nervous system (brain and nerve cord) during the late embryonic stage. | | 14646593
 |