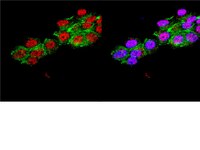
Indolactam V/GLP-1-mediated differentiation of human iPS cells into glucose-responsive insulin-secreting progeny.
Thatava, T; Nelson, TJ; Edukulla, R; Sakuma, T; Ohmine, S; Tonne, JM; Yamada, S; Kudva, Y; Terzic, A; Ikeda, Y
Gene therapy
18
283-93
2010
Zobrazit abstrakt
Nuclear reprogramming of somatic tissue enables derivation of induced pluripotent stem (iPS) cells from an autologous, non-embryonic origin. The purpose of this study was to establish efficient protocols for lineage specification of human iPS cells into functional glucose-responsive, insulin-producing progeny. We generated human iPS cells, which were then guided with recombinant growth factors that mimic the essential signaling for pancreatic development. Reprogrammed with four stemness factors, human fibroblasts were here converted into authentic iPS cells. Under feeder-free conditions, fate specification was initiated with activin A and Wnt3a that triggered engagement into definitive endoderm, followed by priming with fibroblast growth factor 10 (FGF10) and KAAD-cyclopamine. Addition of retinoic acid, boosted by the pancreatic endoderm inducer indolactam V (ILV), yielded pancreatic progenitors expressing pancreatic and duodenal homeobox 1 (PDX1), neurogenin 3 (NGN3) and neurogenic differentiation 1 (NEUROD1) markers. Further guidance, under insulin-like growth factor 1 (IGF-1), hepatocyte growth factor (HGF) and N-[N-(3,5-Difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester (DAPT), was enhanced by glucagon-like peptide-1 (GLP-1) to generate islet-like cells that expressed pancreas-specific markers including insulin and glucagon. Derived progeny demonstrated sustained expression of PDX1, and functional responsiveness to glucose challenge secreting up to 230 pM of C-peptide. A pancreatogenic cocktail enriched with ILV/GLP-1 offers a proficient means to specify human iPS cells into glucose-responsive hormone-producing progeny, refining the development of a personalized platform for islet-like cell generation. Celý text článku | Immunofluorescence | 21048796
 |
Expression in murine teratocarcinoma F9 cells of transcription factors involved in pancreas development.
O'Driscoll L, Gammell P, Clynes M.
Transplantation proceedings
36
1151-8
2004
Zobrazit abstrakt
BACKGROUND: Although it has been established that formation and functional differentiation of the pancreas from embryonic endoderm is associated with activation/inactivation of many genes controlled by specific sets of transcription factors, the role and activation sequence of individual transcription factors has not yet been fully elucidated. This study sought to differentiate a murine teratocarcinoma cell line, F9, to endodermal-like cells and, subsequently; to investigate the effects of regulated expression of transcription factors in pancreas development. METHODS: Following differentiation using retinoic acid and db cAMP (RAC), resulting F9 cells (F9-RAC) were transfected with cDNAs for PDX-1, ngn3, beta 2/NeuroD (beta 2), and Nkx2.2, singly or in combination. Expression of these transcription factors was investigated using RT-PCR and immunofluorescence techniques. RT-PCR analysis was used to assess the subsequent effects of expression of these factors on endogenous genes related to pancreas development. RESULTS: Regulated differentiation of F9 cells generated endodermal-like cell types. Following transfection, PDX-1, ngn3, beta 2, and Nkx2.2 were expressed in F9-RAC cells, with their proteins localized mainly in cellular nuclei. Expression of these factors apparently did not affect the endogenous expression of preproinsulin, PDX-1, beta 2, Isl1, Pax4, Pax6, Sonic hedgehog, and Indian hedgehog. CONCLUSION: This study describes the successful transient expression of transcription factors related to pancreas development, following directed differentiation of F9 cells to endoderm-like cells, and shows that treatment of F9 cells with a combination of RAC causes up-regulation of genes relevant to pancreatic development. The lack of further effect of regulated transcription factor expression on these genes may suggest that parietal endoderm- like cells derived from F9 cells is not the optimal lineage from which to develop beta cells. It may be useful to include F9-derived visceral endoderm in future studies. | | 15194401
 |